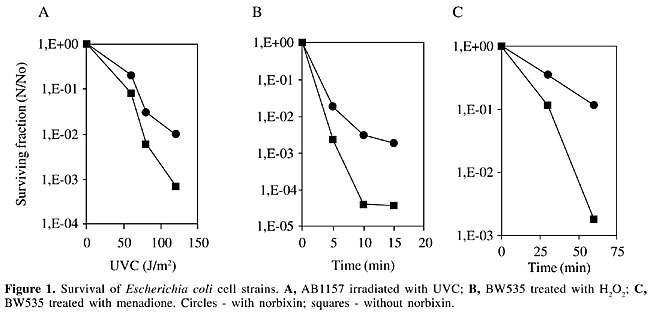
ABSTRACT. Carotenoids are 40-carbon molecules with conjugated double bonds, making them particularly effective for quenching free radicals. They have always been believed to possess anticancer properties, which could be due to their antioxidant potential. Norbixin is an unusual dicarboxylic water-soluble carotenoid present as a component in the pericarp of the seeds of Bixa orellana L. (from the Bixaceae family), a tropical shrub commonly found in Brazil. The main carotenoids present in these seeds, bixin and norbixin, form a coloring material, known as annatto, which is mainly used in the food industry. As annatto is only used as a coloring material, most studies of annatto pigments have focused on the determination of annatto levels in food. However, little attention has been given to the biological properties of bixin and norbixin. We evaluated the effect of norbixin on the response of Escherichia coli cells to DNA damage induced by UV radiation, hydrogen peroxide (H2O2) and superoxide anions (O2.-) and found that norbixin protects the cells against these agents. Norbixin enhanced survival at least 10 times. The SOS induction by UVC was inhibited 2.3 times more when cells were grown in the presence of norbixin. We also found that norbixin has antimutagenic properties, with a maximum inhibition of H2O2-induced mutagenic activity of 87%, based on the Salmonella mutagenicity test. Key words: Annatto pigments, Antigenotoxicity, Antimutagenicity, Norbixin INTRODUCTION The seeds of Bixa orellana L., a shrub native to tropical America, are a rich source of orange-red pigments that have been widely used by the food coloring industry. These pigments are commercially known as annatto (E160b), and their main coloring component is bixin (C25H30O4), an unusual carotenoid having a free carboxyl and an esterified carboxyl as end groups. Currently, the allowable daily intake for annatto is 0-2.5 mg kg body weight-1 day-1 (for a preparation containing 2.6% carotenoids expressed as bixin) and 0-0.065 mg kg body weight-1 day-1, when it is expressed as the oure pigment (JECFA, 1982). Approximately 80% of the pigments present in annatto seeds correspond to bixin (Presto and Rickard, 1980); more recently several other minor carotenoids have been isolated and identified (Mercadante et al., 1997a,b, 1999). Annatto pigments are considered not to be genotoxic, based mainly on in vitro screening data (Sasaki et al., 1980; Haveland-Smith, 1981; Ishidate et al., 1984; Fujita et al., 1988). We recently demonstrated that fibroblasts treated with norbixin in vitro were rendered either resistant or susceptible to DNA damage induced by hydrogen peroxide, as measured by the comet assay (Kovary et al, 2001). The in vivo genotoxic potential of the annatto pigments was also investigated in our laboratory and we observed that norbixin ingestion did not induce any detectable DNA breakage in liver and kidney (Fernandes et al., 2002). Naturally occurring antioxidants, such as carotenoids, have been extensively studied for their potential in reducing the risk for cancer and other chronic diseases (Collins, 2001). We evaluated the effect of norbixin on the response of Escherichia coli cells to DNA damage that has been induced by UV radiation, hydrogen peroxide (H2O2) or superoxide anions (O2.-). We also tested, using the Salmonella mutagenicity test, whether norbixin has a potential antimutagenic activity against an oxidative mutagen (H2O2). MATERIAL AND METHODS Isolation of norbixin Norbixin present in the pericarp of the annatto seeds was extracted with three volumes of CH3CH2OH:H2O(93:7, v/v), at 37°C for several hours, with vigorous shaking following the procedure described by Kovary et al. (2001). Norbixin levels were determined by spectrophotometry (Shimadzu UV-160A spectrophotometer, Japan). Survival experiments We used strains derived from E. coli K12 (BW535 (xthA nfo nth), (AB1157 (wild-type) and PQ65 (sfiA::lacZ). Cells were grown overnight at 37°C in LB rich medium (Miller, 1972), containing, or not, 2 mM norbixin. A starting inoculum was taken from these cultures and grown until the exponential phase in the same medium, containing, or not, 2 mM norbixin. The cells were then centrifuged three times, resuspended in saline (0.9% NaCl) and irradiated with different doses of UVC, or treated with 2.5 mM H2O2 (30% Perhidrol, Merck-Brazil) for 15 min or with 10 mM menadione for 60 min. Samples (0.1 ml) were collected, appropriately diluted in saline, and spread onto LB medium solidified with 1.5% agar. The colonies were counted after overnight incubation at 37°C. The standard deviations did not exceed 5% for all points. SOS chromotest and antimutagenicity assay The induction of b-galactosidase expression by UV radiation was measured in E. coli PQ65, as previously described (Asad et al., 1997, reviewed by Quilardet and Hofnung, 1993), and the induction factor was calculated, based on Goerlich et al. (1989). The result was considered positive when the increase in the induction factor was more than 1.5, combined with an increase in the b-galactosidase activity. Antimutagenicity was measured with Salmonella typhimurium TA102 cells using Ames test procedures (Kovary et al., 2001). This strain is particularly responsive to oxidative and alkylating mutagens, and it detects active forms of oxygen. After incubation for 72 h at 37°C, the number of reverting his+ bacteria colonies was scored. The data shown correspond to the mean of two independent determinations. The standard error of the mean did not exceed 15%. RESULTS AND DISCUSSION Norbixin (2 mM) gave protection against UVC radiation in wild-type cells (Figure 1A). Besides, we also observed protection against H2O2 (Figure 1B) and menadiona (an O2.- producer) (Figure1C) in the xthA-nfo-nth mutant. The data obtained with UV are in agreement with those reported by Tuveson and Sandmann (1993).
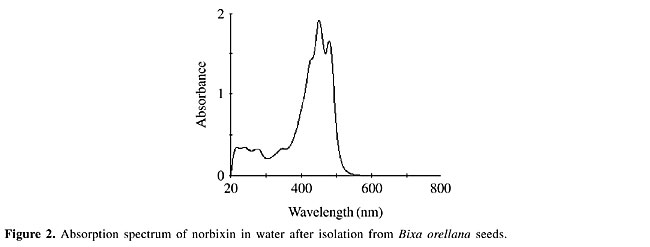
The typical absorption spectra of norbixin in water displays maximum absorptions at 453 and 482 nm (Kovary et al., 2001) (Figure 2). As norbixin also gave absorption at the shorter wavelengths of UVC radiation (<300 nm), norbixin-induced protection against far-UV could be due to UV absorption by norbixin. However, this effect was dose-dependent, and the protection was only evident at high UV-doses (Figure 1A).
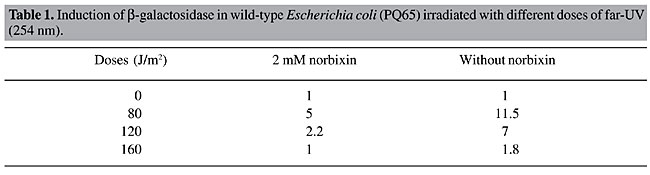
The SOS response plays a central role in the response of E. coli to genotoxic agents (Quilardet and Hofnung, 1993). We studied the effect of norbixin on the genotoxicity induced by UVC radiation using an E. coli strain harboring a lacZ::sfiA fusion (PQ65) in the SOS chromotest assay. We observed that SOS induction by UVC was inhibited when cells were grown in medium containing norbixin (Table 1). These data suggest that SOS induction by UVC radiation is not only due to adducts formed between neighboring pyrimidines (Friedberg et al., 1995), but is also due to oxidative DNA damage.
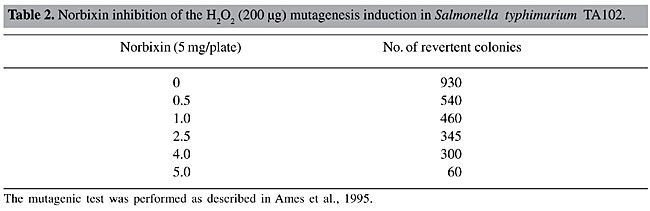
Norbixin was also found to have antimutagenic properties, with a maximum inhibition of H2O2-induced mutagenic activity of 87% (Table 2). The mutagenic activity of norbixin alone (without H2O2) was also tested and no mutagenic activity was detected (data not shown).
The carotenoids, in the form of b-carotene, are known as powerful quenchers/scavengers of singlet O2, which can be formed during lipid peroxidation (Halliwell and Gutteridge, 1999). Singlet O2 has been suggested to mediate the toxicity of O2.- (Halliwell and Gutteridge, 1999). Based on our observations, we suggest that norbixin (an annatto pigment) can be a scavenger of singlet O2, preventing the generation of O2.- and H2O2. ACKNOWLEDGMENTS Research supported by FAPERJ, CNPq, and SR2-UERJ. REFERENCES Ames, B.N., MacCann, J. and Yamasaki, E. (1995). Methods for detecting carcinogens and mutagens with Salmonella/mammalian-microsome mutagenicity test. Mutat. Res. 367: 203-208. Asad, L.M.B.O., Asad, N.R., Silva, A.B., Almeida, C.E.B. and Leitão, A.C. (1997). Role of RecA and OxyR proteins in the repair of DNA iron-independent lesions induced by hydrogen peroxide in Escherichia coli. Biochimie 79: 359-364. Collins, A. (2001). Carotenoids and genomic stability. Mutat. Res. 475: 1-28. Fernandes, A.C.S., Almeida, C.A., Albano, F., Laranja, G.A.T., Felzenszwalb, I., Lage, C.L.S., de Sa, C.C.N.F., Moura, A.S. and Kovary, K. (2002). Norbixin ingestion did not induce any detectable DNA breakage in liver and kidney but caused a considerable impairment in plasma glucose levels of rats and mice. J. Nutr. Biochem. 13: 411-420. Friedberg, E., Walker, G. and Sied, W. (1995). DNA Repair and Mutagenesis. Academic Press, Inc., New York, NY, USA. Fujita, H., Nakano, M. and Sasaki, M. (1988). Mutagenicity test of food additives with Salmonella typhimurium TA97 and TA102. Kenkyu Nenpo-Tokyo-Toritsu Elsei Kenkynsho 39: 343-350. Goerlich, O., Quilardet, P. and Hofnung, M. (1989). Induction of the SOS response by hydrogen peroxide in various Escherichia coli mutants with altered protection against oxidative DNA damage. J. Bacteriol. 171: 6141-6147. Halliwell, B. and Gutteridge, J.M.C. (1999). Free Radicals in Biology and Medicine. Oxford University Press, New York, NY, USA. Haveland-Smith, R.B. (1981). Evaluation of the genotoxicity of some natural food colours using bacterial assays. Mutat. Res. 91: 285-290. Ishidate, M., Sofuni, K., Yoshikawa, K., Hayashi, M., Nohmi, M. and Matsuoka, A. (1984). Primary mutagenicity screening of food additives currently used in Japan. Food Chem. Toxicol. 22: 623-636. JECFA (1982). Evaluation of certain food additives and contaminants. Twenty-six Report of the Joint FAO/WHO Expert Commitee on Food Additives. Technical Report series No. 683. Kovary, K., Louvain, T.S., Silva, M.C.C., Albano, F., Pires, B.B.M., Laranja, G.A.T. Lage, C.L.S. and Felzenszwalb, I. (2001). Biochemical behaviour of norbixin during in vitro DNA damage induced by reactive oxygen species. Br. J. Nutr. 85: 431-440. Mercadante, A.Z., Steck, H. and Pfander, H. (1997a). Isolation and structure elucidation of minor carotenoids from annatto (Bixa orellana L.) seeds. Phytochemistry 46: 1379-1383. Mercadante, A.Z., Steck, H. and Pfander, H. (1997b). Isolation and identification of new apocarotenoids from annatto (Bixa orellana) seeds. J. Agric. Food Chem. 45: 1050-1054. Mercadante, A.Z., Steck, H. and Pfander, H. (1999). Three minor carotenoids from annatto (Bixa orellana) seeds. Phytochemistry 52: 135-139. Miller, J.H. (1972). A Short Course in Bacterial Genetics: A Laboratory Manual and Handbook for Escherichia coli and Related Bacteria. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, USA. Preston, H.D. and Rickard, M.D. (1980). Extraction and chemistry of annatto. Food Chem. 5: 47-56. Quilardet, P. and Hofnung, M. (1993). The SOS chromotest: a review. Mutat. Res. 297: 235-279. Sasaki, M., Sugimura, K., Yoshida, M. and Abe, S. (1980). Cytogenetic effects of 60 chemicals on cultured human and Chinese hamster cells. Senshokutai 20: 574-584. Tuveson, R.W. and Sandmann, G. (1993). Protection by cloned carotenoid genes expressed in Escherichia coli against phototoxic molecules activated by near-ultraviolet light. Methods Enzymol. 214: 323-330. |
|