DNA extraction from bristles and quills of Chaetomys subspinosus (Rodentia: Erethizontidae) using a novel protocol C.G. Oliveira1, R.A. Martinez1,2 and F.A. Gaiotto1,3
Genet. Mol. Res. 6 (3): 657-666 (2007) ABSTRACT. DNA extraction protocols are as varied as DNA sources. When it comes to endangered species, it is especially important to pay attention to all details that ensure the completion of the study goals and effectiveness in attaining useful data for conservation. Chaetomys subspinosus (Rodentia: Erethizontidae) is a secretive arboreal porcupine endemic to certain ecosystems of the Brazilian Atlantic Forest. A multidisciplinary study (including genetic data) was performed to create a management plan for the conservation of this species. Individuals from natural populations of the states of Bahia, Espírito Santo and Sergipe were sampled. To obtain a reliable and abundant amount of starting material, non-destructive methods were tested, extracting DNA from the bristles and quills that comprise most of this animal’s hide. This method has also been innovative in adapting a DNA extraction protocol traditionally used for plants. Digestion using proteinase K was followed by protein precipitation with CTAB, a chloroform-isoamyl alcohol cleaning and DNA precipitation with isopropyl alcohol. This protocol supplies good-quality DNA for genetic analysis with molecular markers based on PCR. Key words: Bristle/hair DNA extraction, Chaetomys subspinosus, Threatened species, Molecular markers, Atlantic Forest, Brazil INTRODUCTION The thin-quill porcupine, Chaetomys subspinosus Olfers 1818 (Rodentia: Erethizontidae) is a medium-size rodent endemic to the northeastern Atlantic Forest in Brazil. It was once considered a common species distributed from the state of Rio de Janeiro to the state of Sergipe. Its habitat is restricted to certain patches of vegetation that form a closed canopy of broad tree tops tangled with bromeliads and woody lianas with a complex understory of secondary species, known as "restinga", and its surrounding environments (Santos et al., 1987; Fonseca et al., 1994). However, environmental degradation is causing a rapid loss of their habitat, affecting natural populations and causing their steady decline. Currently, it is found only in the states of Sergipe, Espírito Santo and Bahia (Giné G, personal communication). Erethizontidae are characterized by having a mixture of thin hair and bristles or quills, which are hard collagenous formations of variable length, with the same tissue origin as normal thin hairs. In C. subspinosus, bristles dominate over hairs, forming a brown-coated mane of thick and stiff short brown spines, with few thin hairs underneath (Santos et al., 1987; Chiarello et al., 1997). This species is elusive, and due to their specific biological requirements, their populations are vulnerable and difficult to estimate. Population size is a basic ecological attribute and its estimation of central importance in conservation and wildlife management. Reliable estimates are necessary to assess the conservation status of any species. However, taking a census in a population is often difficult, especially in rare, cryptic, small, arboreal, and fossorial species (Frantz et al., 2004). Non-invasive/non-destructive genetic sampling has been emerging in recent years as an important tool for molecular genetic studies of free-ranging mammals (Taberlet et al., 1999) as well as for animal abundance estimations (Piggott and Taylor, 2003). DNA can be extracted from sources such as feces and hair follicles without the need to capture the target animal (Piggott and Taylor, 2003). This is an attractive alternative as it requires less skill, time and money compared to collecting blood or biopsy samples (Goossens et al., 1998). There are myriads of known methods to analyze molecular diversity (Frankham et al., 2002). Each type of method has its own usefulness and applications, and researchers have traditionally opted for one or another when working with plant or animal DNA. Williams et al. (1990) described a nuclear DNA amplification using random primers (random amplified polymorphic DNA, RAPD) to optimize genetic mapping in plants. It is basically a polymerase chain reaction (PCR), with several modifications introduced to amplify the polymorphic marker quantity and to extend its potential use. The principal modifications were the use of a unique primer instead of a primer pair that anneals at arbitrary sequence sites in the genome to start the DNA fragment synthesis. The need for information about the nucleotide sequences that flank the DNA sequence of interest becomes unnecessary. Primer size is smaller (10 bp versus generally 20 bp in a sequence-specific PCR) and the annealing temperature is reduced to approximately 35ºC (Ferreira and Grattapaglia, 1998). RAPD markers can be dispersed throughout the whole genome, with no evidence of grouping in specific regions (Williams et al., 1990). Some of the best-known characteristics of RAPD markers are: i) differentiation between polymorphic individuals, ii) low cost, iii) speed, iv) ease of use, v) consistency, and vi) multiplex capacity (the ability to sample the genome in various loci at the same time). However, they may be considered disadvantageous compared to other molecular markers commonly used (i.e., SSR) because of their dominant character and binary interpretation (band presence or absence) (Ferreira and Grattapaglia, 1998). They have been scarcely used in animal studies due to supposed repeatability difficulties and ambiguous results (Frankham et al., 2002; Avise, 2004). However, besides the many publications using RAPDs with dozens of plant species, important results obtained with animal DNA have been found in recent literature (Cooper, 2000; Chiappero and Gardenal, 2001; Gonzalez-Ittig et al., 2002). Certain regions of animal mitochondrial DNA (mtDNA) are excellent tools for genetic variability analysis because of their considerable variation within and among populations (Parker et al., 1998). Success of the mtDNA sequence in any variability study is due to several characteristics: a) compact gene packaging, with little intergenic non-coding nucleotides and some stacked nucleotides between genes (Cantatore and Saccone, 1987); b) absence of recombination (Clayton, 1992); c) overwhelming predominance of maternal heredity (Kondo et al., 1990); d) sequence evolution, faster than nuclear sequences due to repair inefficiency (Brown et al., 1979), and e) the existence of multiple copies in the cell (Robin and Wong, 1988). Overall, the goal of this report was to offer a new protocol for DNA extraction from small, non-destructive samples of porcupine spines/quills, rarely reported as starting DNA material. MATERIAL AND METHODS Biological materialStudy material came from individuals captured within forest fragments in three northeastern Brazilian states: Bahia (BA) (districts of Itacaré, Una, Uruçuca, Ilhéus, Salvador, Amargosa, and Cachoeira), Sergipe (SE) (district of Santa Luzia do Itanhi) and Espírito Santo (ES) (Paulo César Vinha State Park). Three populations were considered, one for each state, totaling 27 individuals; 19 from BA, two from SE and six from ES. Captures were performed between March 15, 2005 and April 15, 2005. Low vegetation habitats and possible resting sites such as entangled lianas, typical understory formations known as "tabocais", tree trunks and bromeliads were searched, as they pose as potential microhabitats for these individuals (Giné G, personal communication). After locating an animal and its correct identification as C. subspinosus, the tree was climbed and the animal was removed from the vegetation. It was held by the neck with leather gloves to put on a radio collar for population studies, and quills (and the thin hairs underneath) were then collected by plucking them gently. Samples were wrapped in tinfoil and kept dry until they were delivered to the laboratory, where DNA extraction took place as soon as possible after their arrival. DNA extractionFor DNA extraction, most of the quill was discarded, leaving the bulb and around 2-3 mm of the remaining hair. The CTAB (cationic hexadecyl trimethyl ammonium bromide) protocol from Doyle and Doyle (1987), originally used for plants, was modified by adding proteinase K, normally used for animal tissues. A total of 50 hair bulbs were left for at least 4 h at 65ºC in 50 µL proteinase K (10 mg/mL) and 500 µL lysis buffer solution (1 M Tris-HCl, pH 8.0, 5 M NaCl, 10% SDS, 0.5 M EDTA), using a water bath as a heat source because it is considered more stable and gentle for enzyme degradation than a dry oven (Sambrook et al., 1989). When the hair was visibly degraded and hair bulbs barely noticeable, 1 µL 0.2% β-mercaptoethanol, which hinders DNA oxidation during the extraction, and 350 µL CTAB buffer (2% CTAB, 1 M NaCl, 20 mM EDTA, 1000 mM Tris-HCl, pH 8.0, 1% polyvinylpirrolidone) were added directly to the tube, and left for more 2 h in the same solution. After cooling at room temperature, 300 µL chloroform/isoamyl alcohol 24:1 was added and the tube was inverted vigorously 100 times, followed by a 10-min centrifugation at 12,000 rpm. The supernatant was transferred to another Eppendorf tube with 200 µL isopropanol, gently mixed and left in the freezer at -20ºC for 1 h. After precipitation time in the freezer, the sample was centrifuged for 15 min at 12,000 rpm, this time keeping the pellet and adding 400 µL 70% ethanol. After this first wash, the pellet was centrifuged for 10 min at 12,000 rpm, followed by two or three more washes with ethanol until the pellet appeared creamy and homogeneous. The tube was left uncapped opened at room temperature until there was no sign of remaining ethanol. The final step was the addition of 20 µL TE + RNAse (10 µg/mL) followed by an incubation at 37ºC for 30 min. To confirm DNA extraction, a comparison was made with known amounts of λ DNA run along with the samples on 1% agarose gel, and RAPD and mtDNA primers were used in PCR. Other extraction procedures were performed with the same samples to compare the DNA quality and quantity obtained using our extraction protocol. These included: i) phenol-chloroform extraction (Sambrook et al., 1989); ii) Chelex 100 resin (Biorad) (Walsh et al., 1989), following the method described by Ascunce et al. (2002); iii) commercial reagent Brazol®, following the manufacturer’s instructions, and iv) modified Brazol® protocol with an overnight preincubation with proteinase K (10 mg/mL). DNA quantificationDNA quantification was compared with known standards of λ phage DNA (10, 50 and 100 ng/µL). Volumes of 1 µL of each known λ DNA concentration and 2 µL of each extracted DNA sample in 2 µL 6X carrying buffer (1.6 M sucrose, 1.6 mM bromophenol blue) were applied side by side in gel lanes. Electrophoresis was performed on 1.5% agarose gel in 1X TBE (8.9 mM Tris, 88 mM boric acid, 10 mM EDTA). DNA was visualized under UV light after the addition of ethidium bromide (0.25 mg/mL) and diluted in Milli-Q water to a concentration of 2.5 ng/µL for amplification with RAPD markers and mtDNA. Amplification conditionsRandom amplified polymorphic DNAAfter RAPD repeatability conditions were ensured (Oliveira, 2005), a final 13-µL volume of PCR was performed, with 1.3 µL of a 10X buffer (1 M Tris, pH 8.3, 1 M MgCl2, 50 mM KCl), 1.3 µL 2.5 mM dNTPs, 1 µL 2.5 mg/mL BSA, 3 µL 5 ng/µL primer, 0.2 µL 5 U/µL Taq polymerase, 3.42 µL sterile water, and 7.5 ng DNA, using a Perkin-Elmer GeneAmp® PCR System 9700. Reactions had an initial denaturation step at 92ºC for 2 min, followed by 40 amplification cycles of 1 min at 92ºC, 1 min at 35ºC and 2 min at 72ºC with final 5-min extension step at 72ºC. PCR products were visualized on 1.5% agarose gels in 1x TBE under UV light and were photographed with a Kodak EDAS 24.0 photodocumentation system. A 1-kb ladder (0.66 ng per gel; Invitrogen) was used as a molecular weight marker. Mitochondrial DNAAmplification of the D-loop region of the mtDNA was performed using the universal primers designed for mammals, L-15996 (Kocher et al., 1989) and HD2 (Nagata et al., 1998). Reactions were carried out with 25 ng DNA, 2.5 µL 10X PCR buffer, 1.75 µL MgCl2, 1 µM of each primer, 4 µL 2.5 mM dNTPs, 1 U DNA Taq polymerase, and Milli-Q water in a final volume of 25 µL. Amplification conditions were: an initial denaturation step at 94ºC for 5 min, followed by 40 amplification cycles of 1 min at 94ºC, 1 min at 47ºC, and 1 min and 20 s at 72ºC with a final 10-min extension step at 72ºC. PCR products were visualized on 1% agarose gels in 1x TBE, with 10 µL of a 1-kb ladder (Invitrogen) as a molecular weight marker. Gels were photographed under UV light with a Kodak EDAS 24.0 photodocumentation system. RESULTS AND DISCUSSION This study represents the first application of the CTAB DNA extraction protocol for non-destructive samples, such as hairs. It has the additional asset of being one of the few DNA extraction protocols reported for modified hairs, such as bristles or quills. The original CTAB protocol (Doyle and Doyle, 1987) was designed for plant DNA, and yielded poor results when first tested on hair DNA extraction, with no visible DNA band on 1.5% agarose test gel, where the presence of DNA was confirmed only through RAPD amplification (Table 1), which is more time consuming and expensive. The Chelex-100® protocol according to modifications suggested by Ascunce et al. (2002) and the commercial reagent Brazol® combined with proteinase K digestion both showed poor absolute DNA yields with visible DNA amplification only after an RAPD reaction (Figure 1). Traditional phenol/chloroform DNA extraction (Sambrook et al., 1989) was used as a positive control test, producing results comparable to our CTAB protocol. However, the CTAB modified protocol was preferred because of its simplicity, reduced toxicity and relatively low price (Table 1). An additional test was performed with 50% less CTAB than the original protocol, and the same amount of hair bulbs, to ensure that DNA was not lost due to an excess of reagent, which actually had a significant effect, for amplification was possible using only half the amount of CTAB (Table 1). The addition of a pre-digestion step with proteinase K plus the addition of β-mercaptoethanol to CTAB washing makes this protocol effective with low-yield DNA samples, such as hair, because proteinase K efficiently degrades all proteins, while β-mercaptoethanol guarantees DNA protection from oxidation during the following purification steps. With a 4-h exposure to proteinase K at 65ºC, followed by β-mercaptoethanol/CTAB extraction, yields were approximately 100 ng/µL DNA, which can be stored at 4ºC (Figure 1). 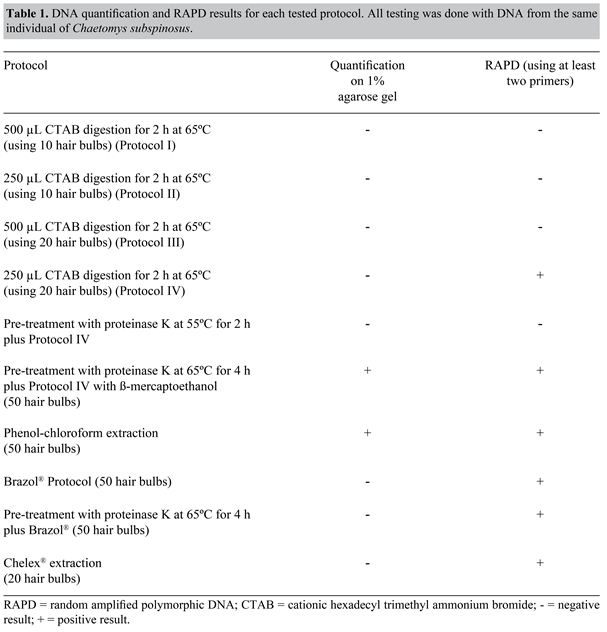
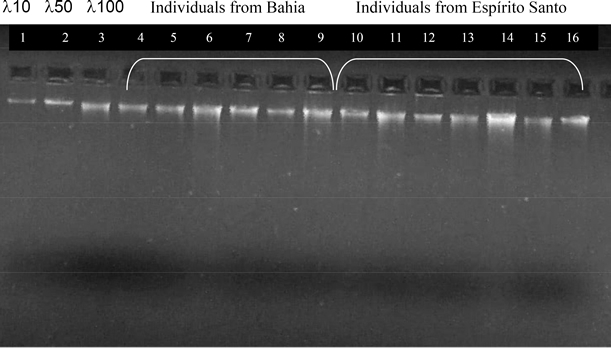
Figure 1. DNA quantification for 14 samples of Chaetomys subspinosus extracted using the proteinase K plus CTAB protocol reported here for the first time. The first three lanes represent known molecular weight markers (λ phage DNA) of 10, 50 and 100 ng/µL, respectively. Lanes 4 to 9, individuals from Bahia, lanes 10 to 16, individuals from Espírito Santo. For samples difficult to obtain, such as those from wild animals with low population numbers (which is the case for C. subspinosus), storage of starting material may represent the key to successful data collection, because inadequate storage can spoil any DNA sample, rendering it useless (Ferreira and Grattapaglia, 1998). DNA extraction has been successfully reported for hairs even after years of storage (Hummel and Hummel, 1994). However, this was not the case for C. subspinosus, where time between hair plucking and DNA extraction was directly related to a successful yield. Therefore, to obtain maximum results, hair was treated upon arrival in the laboratory. This ensured a sufficient amount of DNA to complete the analyses and store in a national DNA bank, as is required by Brazilian law, and no hair was kept for re-extraction. We believe that metabolic peculiarities of C. subspinosus may be associated with rapid degradation of material upon extraction. Another possibility may be related to handling conditions of the samples in the field, which could have led to DNAse contamination and subsequent DNA degradation in a short period of time. Conversely, all protocols were tested in long-term stored spines of a close relative, the yellow-spined porcupine Sphiggurus insidiosus (Rodentia: Erethizontidae), showing no DNA loss or degradation (Oliveira C, unpublished results). Since few reports on DNA extraction for representatives of the New World porcupines, family Erethizontidae, have been found, a series of protocols had to be tested based on protocols for hair, fresh tissue, blood, or cultured cells (Sambrook et al., 1989). Since C. subspinosus is classified as “vulnerable” by the World Conservation Union (IUCN, 2001), and is absent from most captive institutions in Brazil or overseas, we depended solely on the scanty samples brought from the wild, even with a low chance for repeating a lost sample. CTAB protocol seemed optimal to test because it required no previous tissue preparation and could be done as soon as the sample arrived in the lab. However, the proteinase K incubation period turned out to be essential for good DNA yields, and since part of the analysis was to be carried out using RAPD primers, good reproducibility of every step of the protocol was necessary to ensure repeatability of the results, which was demonstrated elsewhere (Oliveira, 2005) (Figure 2). mtDNA amplification, specifically of 476 bp of the D-loop region using primers L-15996 (Kocher et al., 1989) and HD2 (Nagata et al., 1998) (Figure 3), was also successful, demonstrating the potential of the extraction protocol and the good quality of the DNA for other kind of analyses.
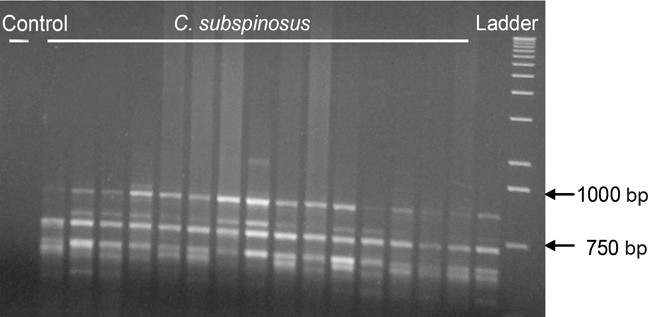
Figure 2. RAPD gel using primer OPR-08 for 16 individuals of Chaetomys subspinosus from the States of Espírito Santo, Bahia and Sergipe. The first lane represents the negative control and the extreme right column represents a 1-kb ladder (Invitrogen).
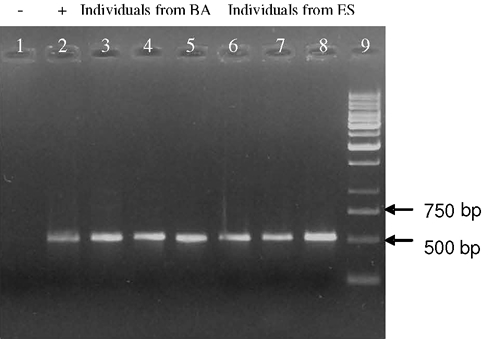
Figure 3. mtDNA D-loop (approximately 500 bp) from Chaetomys subspinosus using primers L-15996 and H-HD2. Lane 1, Negative control; lane 2, positive control (human DNA); lanes 3 to 5, C. subspinosus from Bahia (BA), lanes 6 to 8, C. subspinosus from Espírito Santo (ES), and lane 9, a 1-kb ladder (Invitrogen). Due to the nature of the starting material, which could become contaminated with contemporary animal DNA during extraction, caution was taken to guarantee specific product amplification from the study animal and not other contamination sources such as fungus, microbes, humans, plants, or ectoparasites, as well as to avoid contamination by pre-amplified products (Hummel and Hummel, 1994). DNA isolation was performed in a specific work station, where only C. subspinosus’ material was studied. Safety and authenticity criteria, set up for older DNA by Handt et al. (1994), were observed during our laboratory procedures, including: 1) physical separation in the laboratory to extract and amplify the DNA; 2) DNA contamination from researcher or other amplified products was prevented, and appropriate clothes, equipment and reagents exclusive for extraction, and workbench sterilization were used; 3) a control amplification was established to detect potential contamination with human DNA during the extraction leading to amplification of samples without DNA (negative control) or with DNA other than that of porcupine (Figure 3). Moreover, mtDNA sequences from C. subspinosus were aligned using other rodent sequences retrieved from PubMed using the BLAST program (http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/genom_table_cgi), showing more similarity with the mtDNA of other rodents such as Agouti paca, Cryptomys damarensis, Heliophobius argenteocinereus, and Cavia porcellus, and less likeness with other animal or eukaryote DNA. Thus, contamination free, C. subspinosus DNA was confirmed and used as sole source for our subsequent amplifications. Finally, we wish to stress the importance of this efficient, quick and inexpensive protocol for non-destructive samples, especially due to the nature of our starting material (modified hairs, such as bristles or quills). We must also keep in mind that every sample taken from wild animals, particularly those under any threat of extinction, takes an enormous amount of time and budget consumption, genetics being only a small part of the multidisciplinary conservation efforts. Furthermore, any success in simple and repeatable data gathering is a major step towards the reduction of the risk of extinction of this inestimable asset that is our endangered fauna. ACKNOWLEDGMENTS This research is part of the project “Elaboration of a management plan for Chaetomys subspinosus” which was supported by FNMA (Fundo Nacional do Meio Ambiente). We wish to thank the project coordinator Dr. Deborah Faria for gathering an excellent team. C.G. Oliveira is thankful to FAPESB for the MSc scholarship. We are also grateful to UESC for allowing all experimental procedures to be conducted and to IBAMA which granted legal permits for all parts of this study. Finally, thanks go to Rivelino Galvão, Mr. Toninho and MSc. Gastón A.F. Giné for providing samples from their field studies. REFERENCES Ascunce MS, Hasson E and Mudry MD (2002). Description of the cytochrome c oxidase subunit II gene in some genera of New World monkeys (primates, Platyrrhini). Genetica 114: 253-267. Avise J (2004). Phylogeography: the history and formation of species. Harvard University Press, Cambridge. Brown WM, George M Jr and Wilson AC (1979). Rapid evolution of animal mitochondrial DNA. Proc. Natl. Acad. Sci. USA 76: 1967-1971. Cantatore P and Saccone C (1987). Organization, structure, and evolution of mammalian mitochondrial genes. Int. Rev. Cytol. 108: 149-208. Chiappero MB and Gardenal CN (2001). Inheritance of random amplified polymorphic DNA (RAPD-PCR) markers and their use in population genetic studies of Calomys musculinus (Rodentia, Muridae), the reservoir of Argentine hemorrhagic fever. Hereditas 135: 85-93. Chiarello AG, Passamani M and Zortéa M (1997). Field observations on the thin spined porcupine, Chaetomys subspinosus (Rodentia: Echimyidae). Mammalia 61: 29-36. Clayton DA (1992). Transcription and replication of animal mitochondrial DNAs. Int. Rev. Cytol. 141: 217-232. Cooper ML (2000). Random amplified polymorphic DNA analysis of southern brown bandicoot (Isoodon obesulus) populations in western Australia reveals genetic differentiation related to environmental variables. Mol. Ecol. 9: 469-479. Doyle JJ and Doyle JL (1987). Isolation of plant DNA from fresh tissues. Focus 12: 13-15. Ferreira ME and Grattapaglia D (1998). Introdução ao uso de marcadores RAPD e RFLP em análise genética. EMBRAPA-CENARGEN, Brasília. Fonseca GAB, Rylands AB, Costa CMR, Machado RB, et al. (1994). Livro vermelho dos mamíferos brasileiros ameaçados de extinção. Fundação Biodiversitas, Belo Horizonte. Frankham J, Ballou J and Briscoe F (2002). Introduction to conservation genetics. Cambridge Institute Press, Cambridge. Frantz AC, Schaul M, Pope LC, Fack F, et al. (2004). Estimating population size by genotyping remotely plucked hair: the Eurasian badger. J. Appl. Ecol. 41: 985-995. Gonzalez-Ittig R, Chiappero M, Blanco A, Provensal C, et al. (2002). Accurate identification of three cryptic species of rodents of genus Calomys using RAPD-PCR nd mtDNA RFLP markers. Biochem. Syst. Ecol. 30: 425-432. Goossens B, Waits LP and Taberlet P (1998). Plucked hair samples as a source of DNA: reliability of dinucleotide microsatellite genotyping. Mol. Ecol. 7: 1237-1241. Handt O, Höss M, Krings M and Pääbo S (1994). Ancient DNA: methodological challenges. Experientia 50: 524-529. Hummel S and Herrmann B (Editors) (1994). General aspects of sample preparation. In: Ancient DNA: recovery and analysis of genetic material from paleontological, archaeological, museum, medical, and forensic specimens. Springer Verlag, New York, 59-68. IUCN (2001). IUCN red list categories: Version 3.1. Prepared by the IUCN species survival commmission. IUCN, Gland, Switzerland and Cambridge. Kocher TD, Thomas WK, Meyer A, Edwards SV, et al. (1989). Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc. Natl. Acad. Sci. USA 86: 6196-6200. Kondo R, Satta Y, Matsuura ET, Ishiwa H, et al. (1990). Incomplete maternal transmission of mitochondrial DNA in Drosophila. Genetics 126: 657-663. Nagata J, Masuda R, Kaji K, Kaneko M, et al. (1998). Genetic variation and population structure of the Japanese sika deer (Cervus nippon) in Hokkaido Island, based on mitochondrial D-loop sequences. Mol. Ecol. 7: 871-877. Oliveira CG (2005). Diversidade genética do ouriço preto (Chaetomys subspinosus Olfers 1818, Rodentia: Erethizontidae) para auxiliar na elaboração do seu plano de manejo. Marter’s thesis, Programa de Pós Graduação em Genética e Biologia Molecular, Universidade Estadual de Santa Cruz, Ilhéus. Parker P, Snow A, Shug M, Booton G, et al. (1998). What molecules tell us about populations: choosing and using molecular markers. Ecology 79: 361-382. Piggott MP and Taylor AC (2003). Remote collection of animal DNA and its applications in conservation management and understanding the population biology of rare and cryptic species. Wildl. Res. 30: 1-13. Robin ED and Wong R (1988). Mitochondrial DNA molecules and virtual number of mitochondria per cell in mammalian cells. J. Cell Physiol. 136: 507-513. Sambrook J, Fritsch EF and Maniatis T (1989). Molecular cloning - A laboratory manual. 2nd edn. Cold Spring Habor Laboratory Press, Cold Spring Harbor. Santos IB, Oliver WL and Rylands AB (1987). Distribution and status of two tree porcupines. Chaetomys subspinosus and Sphiggurus insidiousus in south-east Brazil. J. Jersey Wildl. Preservation Trust 24: 43-60. Taberlet P, Waits LP and Luikart G (1999). Noninvasive genetic sampling: look before you leap. Trends Ecol. Evol. 14: 323-327. Walsh P, Metzger D and Higuchi R (1989). Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10: 506-513. Williams JG, Kubelik AR, Livak KJ, Rafalski JA, et al. (1990). DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 18: 6531-6535. |
|