
Cytogenetic characterization of Crenicichla (Pisces, Perciformes, Cichlidae) of the Iguaçu River S.M.H.K. Mizoguchi, A.L.B. Portela-Castro and I.C. Martins-Santos
Genet. Mol. Res. 6 (3): 650-656 (2007) ABSTRACT. Three populations of the genus Crenicichla, namely Crenicichla iguassuensis, Crenicichla sp 1 and Crenicichla sp 2, from the Iguaçu River, were analyzed cytogenetically, and their nucleolus organizer regions, constitutive heterochromatin distribution and chromomycin A3 markings were studied. Karyotype analyses showed a diploid number of 48 chromosomes, made up of 2 metacentric pairs, 2 submetacentric pairs, 7 subtelocentric pairs, and 13 acrocentric pairs for the three Crenicichla species and no sexual chromosome differentiation. Nucleolus organizer regions showed strong interstitial marking on the first chromosome pair, coincident with a constriction presented by Giemsa and positive marking by chromomycin. Although constitutive heterochromatin patterns were also similar, with pericentromeric markings, small differences in the three species could be observed. Crenicichla sp 2 presented some chromosomes with bitelomeric markings absent in Crenicichla iguassuensis and Crenicichla sp 1. Key words: Karyotype, Nucleolus organizer region banding, C banding, Cichlidae, Crenicichla iguassuensis, Perciformes INTRODUCTION From an ecological point of view, the Cichlidae family are highly diversified. They belong to the ichthyofauna of Africa, Madagascar, Central and South America, Mexico, southern India, and Sri Lanka (Kullander and Nijssen, 1989; Kullander, 1998). In spite of the group’s wide geographic distribution, endemic species may be found in the great African lakes (Kullander, 1998). According to Garavello et al. (1997), pronounced ichthyofauna endemism in the Iguaçu River is due to the Iguaçu waterfalls, which geographically isolate the region from the Paraná River. According to Nelson (1994), there are 35 genera and approximately 300 species of cichlids in South America, especially in the Amazon basin. More recent studies, however, have given a total of 52 species, distributed into 14 families, including the 36 already mentioned (Garavello et al., 1997). Sixteen of the 23 families of fish reported in the Paraná River are absent in the Iguaçu River, the most important being the anostomids, serrasalmids, curimatids, doradids, and prochilodontids (Agostinho et al., 1997). Native species, such as Geophagus brasiliensis, Crenicichla iguassuensis and Cichlasoma facetum, and introduced ones, such as Tilapia rendallii are among the cichlids of the Iguaçu River (Garavello et al., 1997). Described by Haseman in 1911, C. iguassuensis has teeth in several series, depressible posterior teeth, normal lips, upper lateral line distant from the dorsal one, and a dark, elongated body. On the other hand, Crenicichla sp, studied by Kullander SO, Pavanelli CS, Lucena CAS and Garavello JC (personal communication), has turgid lips with adipose seams. Lucena and Kullander (1992) analyzed several Crenicichla species of the Uruguay River and described C. tendybaguassu as having big lips similar to Crenicichla sp of the Iguaçu River. A cytogenetic study of the Cichlidae has shown conservative karyotype evolution with regard to diploid number, and all the species of the Crenicichla genus studied until the moment present a diploid number of 2n = 48 chromosomes, as do most species of this family. Enzymatic analysis of 27 loci from Crenicichla sp and C. iguassuensis, failed to find any diagnostic locus that would indicate two distinct species (Renesto et al., 2001). Also, the existence of a third species in the same basin has been proposed. Since this species has intermediate-size lips, its presence would suggest three C. iguassuensis morphotypes in the Iguaçu River. Our objective was to characterize cytogenetically the different forms and help inform about the hypothesis of more than one Crenicichla species in this basin. MATERIAL AND METHODS Cytogenetic analyses were undertaken of 71 individuals (24 females and 20 males of C. iguassuensis, 4 females and 6 males of Crenicichla sp 1, and 8 females and 9 males of Crenicichla sp 2), from the Iguaçu River close to the Salto Caxias Reservoir (25º 32’ 07 S/53º 28’ 59 W) in the state of Paraná, Brazil. Mitotic chromosome preparations were obtained from kidney cells by the air-drying technique described by Bertollo et al. (1978). Constitutive heterochromatin distribution and chromomycin A3 (CMA3) were analyzed according to basic procedures suggested by Sumner (1972) and Schweizer (1976), respectively. The nucleolus organizer regions (NORs) were analyzed by silver nitrate (Ag-NOR) staining, following the method described by Howell and Black (1980). Chromosomal types were identified by the arm-ratio criterion proposed by Levan et al. (1964). RESULTS Cytogenetic analysis of Crenicichla iguassuensis and its morphotypes, Crenicichla sp 1 and Crenicichla sp 2 showed similar karyotypes with the same diploid number of 48 chromosomes, distributed as 2 metacentric (M) pairs, 2 submetacentric (SM) pairs, 7 subtelocentric pairs, and 13 acrocentric pairs and fundamental number = 56 (Figure 1). Detection of NORs in the three forms by silver nitrate impregnation indicated strong marking in the interstitial region in the short arm of the first SM pair (Figure 1), coinciding with the secondary constriction by Giemsa conventional analysis and with marking by CMA3 (Figure 2D). 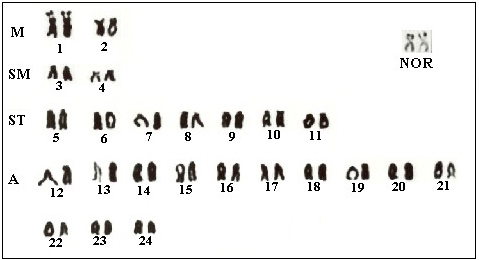
Figure 1. Karyotype of Crenicichla iguassuensis, Crenicichla sp 1 and Crenicichla sp 2. The silver nitrate-nucleolus organizer region (Ag-NOR) chromosome pair is evident. M = metacentric; SM = submetacentric; ST = subtelocentric; A = acrocentric. 
Figure 2. Somatic metaphase of Crenicichla iguassuensis (A) Crenicichla sp 1 (B) and Crenicichla sp 2 (C) evidencing the distribution of the constitutive heterochromatin pattern. Bitelomeric C band in Crenicichla sp 2 (arrows). Somatic metaphase of C. iguassuensis stained with chromomycin A3 (CMA3) representative of the three species (D). Ag-NOR- positive CMA3 band (arrows). Constitutive heterochromatin pattern was also similar in the three morphotypes, with pericentromeric markings for most chromosomes and with weak telomeric bands for some others (Figure 2A-C). However, Crenicichla sp 2 presented certain chromosomes with bitelomeric markings (Figure 2C). NOR had a negative C band (Figure 2A-C). DISCUSSION C. iguassuensis and its two Crenicichla sp forms of the Iguaçu River (Crenicichla sp 1 and Crenicichla sp 2) showed the same diploid number and the same karyotype formula and NOR banding. Although similar, the distribution of constitutive heterochromatin in pericentromeric and telomeric regions was not identical in the three morphotypes. Crenicichla sp 2 presented bitelomeric markings, which were absent in the other morphotypes. Analyses suggested that there were no differences that might characterize them as taxonomically different. Renesto et al. (2001) analyzed 27 enzymatic loci in two of the three C. iguassuensis forms and found a genetic identity of 0.993, coupled with a lack of a diagnostic locus that would differentiate the two Crenicichla morphotypes. Cichlid cytogenetics has shown a conservative karyotype evolution with regard to diploid number; most of the species present 2n = 48 chromosomes and many subtelo-acrocentric chromosomes (Thompson, 1979; Feldberg and Bertollo, 1985a; Martins et al., 1995; Loureiro and Dias, 1999). However, their karyotype formulas show variations, in spite of a constant diploid number (Oyhenart-Perera et al., 1975; Feldberg and Bertollo, 1985a; Martins et al., 1995). Crenicichla species analyzed up to now and other cichlids have also shown a highly conservative chromosome evolution, with a diploid number 2n = 48 chromosomes, and small differences in their karyotype formula (Table 1). This fact may be observed through an analysis of the different species in the group with the same karyotype formula (8 M/SM in Crenicichla sp B; Salgado et al., 1994, and Crenicichla sp, Loureiro and Dias, 1999; 6 M/SM in several species, Table 1). The differences of 6 or 8 M/SM may be a mistake in measurement or differences of chromosome condensation during metaphases under analysis. However, structural rearrangements during the evolutionary process are still a possibility. Consequently, similar karyotypic data in C. iguassuensis, Crenicichla sp 1 and Crenicichla sp 2 corroborate the group’s cytogenetic data and show that morphological differences were not visible at the level of the macrostructure karyotype for differentiating the three Crenicichla morphotypes of the Iguaçu River. 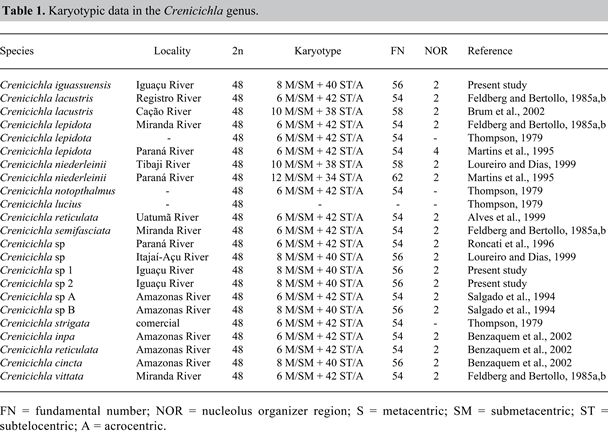
A simple NOR has been found in most Cichlids (Feldberg and Bertollo, 1985b; Martins et al., 1995) with variations in sites on the short or long arm of the first chromosome pair of the complement. However, multiple NORs have been reported in Crenicichla lepidota (Martins et al., 1995) and in Cichlasoma paranaense (Loureiro and Dias, 1998). Sub-terminal secondary constrictions in the first M/SM pair, coinciding with the NOR, have also been reported in most species of the Crenicichla genus, indicating this to be the group’s ancestral chromosome. A terminal NOR of this chromosome pair has been registered only in C. cincta (Benzaquem et al., 2002). Correspondence between silver nitrate marking and CMA3 in the NOR site indicating a GC-rich region, has been reported in the three morphotypes analyzed and constitutes a common characteristic among fish species (Wasko et al., 1996; Portela-Castro, 1999). However, it is not a heterochromatic region, a fact that has not been reported for the other species of the group. Although GC-rich NOR is a common characteristic in cichlids and particularly in the Crenicichla genus, NOR-negative C banding seems to be a marker of Crenicichla from the Iguaçu River. Though the distribution of constitutive heterochromatin regions was not identical in the three morphotypes, this was not indicative of different taxonomic unities. Although morphological differences are a consequence of genetic differences, the environment can affect them, hence morphological environmental differences may occur in the absence of reproductive isolation. Studies on diet and food activity showed that species of the Crenicichla genus are piscivorous and that C. iguassuensis chiefly ingests fish, decapods (Aegla sp) and other invertebrates, whereas Crenicichla sp feeds on fish, decapods (Aegla sp) and a low percentage of detritus and sediments, not detected in C. iguassuensis. Studies on daily food intake show that whereas this species has the highest stomach repletion during the evening-night period, repletion occurs during the day period in Crenicichla sp (Hahn et al., 1997). Diet and feeding activity give important data on species behavior, since feeding source is an important factor in species stabilization and fixing (Agostinho et al., 1997). Although fish with trophic specialization are extant in tropical environments, most have wide feeding flexibility. In our study, cytogenetic data corroborate those found by isozyme analyses (Renesto et al., 2001), which indicate polymorphisms caused by the feeding habits of the three C. iguassuensis forms from the Iguaçu River. However, the group’s highly conservative karyotype may be camouflaging real genetic differences that may characterize them as different taxonomic unities. Molecular studies with RAPD, SPAR and mitochondrial DNA techniques have to be undertaken to define, in a satisfactory way, the taxonomic position of the different Crenicichla forms found in the Iguaçu River reservoir. ACKNOWLEDGMENTS The authors are grateful to CNPq for financial support and to Nupelia for supplying the specimens analyzed. REFERENCES Agostinho AA, Bini LM and Gomes LC (1997). Ecologia de comunidades de peixes da área de influência do Reservatório de Segredo. In: Reservatório de Segredo: bases ecológicas para manejo (Agostinho AA and Gomes LC, eds.). Editora da Universidade Estadual de Maringá, Maringá, 97-109. Alves MN, Santos MNM and Feldberg E (1999). Presença de cromossomos supranumerários em três espécies de Cichlidae da bacia Amozônica. In: XIII Encontro Brasileiro de Ictiologia, São Carlos, SP, Brazil, 155. Benzaquem DC, Silva AM, Porto JIR and Feldberg E (2002). Caracterização cromossômica em três espécies do gênero Crenicichla Heckel, 1840 da Amazônia Central. In: IX Simpósio de Citogenética e Genética de Peixes, Maringá, 96. Bertollo LAC, Takahashi CS and Moreira-Filho O (1978). Cytotaxonomic consideration on Hoplias lacerdae (Pisces, Erythrinidae). Rev. Bras. Genet. 1: 103-120. Brum MJ, Neto AF and Mota LG (2002). Análise cariotípica de Crenicichla lacustris (Perciformes, Cichlidae) do Estado do Rio de Janeiro. In: IX Simpósio de Citogenética e Genética de Peixes, Maringá, PR, Brazil, 97. Feldberg E and Bertollo LAC (1985a). Karyotypes of 10 species of Neotropical cichlideo fish (Pisces, Perciformes). Caryologia 38: 257-268. Feldberg E and Bertollo LAC (1985b). Nucleolar organizing regions in some species of Neotropical cichlid fish (Pisces, Perciformes). Caryologia 38: 319-324. Garavello JC, Pavanelli C and Suzuki HI (1997). Caracterização da ictiofauna do rio Iguaçu. In: Reservatório de Segredo: bases ecológicas para manejo (Agostinho AA and Gomes LC, eds.). Editora da Universidade Estadual de Maringá, Maringá, 61-84. Hahn NS, Fugi R, Almeida VLL, Russo MR, et al. (1997). Dieta e atividade alimentar de peixes do Reservatório de Segredo. In: Reservatório de Segredo bases ecológicas para manejo (Agostinho AA and Gomes LC, eds.). Editora da Universidade Estadual de Maringá, Maringá, 141-162. Howell WM and Black DA (1980). Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia 36: 1014-1015. Kullander SO (1998). A phylogeny and classification of the south American cichlidae (Teleostei, Perciformes). In: Phylogeny and classification of neotropical fishes (Malabarba LR, Reis RE, Vari RP, Lucena ZM, et al., eds.). EDUPUCRS, Porto Alegre, 461-498. Kullander SO and Nijssen H (1989). The cichlids of Surinam. E.J. Brill, Leiden. Levan A, Fredga K and Sandberg AA (1964). Nomenclature for centromeric position on chromosome. Hereditas 52: 201-220. Loureiro MA and Dias AL (1998). Regiões organizadoras de nucléolo (NORs) múltiplas em Cichlasoma paranaense (Pisces, Cichlidae) da região de Guaravera. In: VII Simpósio de Citogenética Evolutiva e Aplicada de Peixes Neotropicais, Londrina, 18. Loureiro MA and Dias AL (1999). Análise citogenética em quatro espécies da família Cichlidae (Pisces, Perciformes). Master’s thesis, Universidade Estadual de Londrina, Paraná. Lucena CAS and Kullander SO (1992). The Crenicichla (Teleostei: Cichlidae) species of the Uruguai River drainage in Brazil. Ichthyol. Explor. Freshwaters 121: 97-160. Martins IC, Portella-Castro ALB and Julio HF Jr (1995). Chromosome analysis of 5 species of the Cichlidae family (Pisces-Perciformes) from the Paraná River. Cytologia 60: 223-231. Nelson JS (1994). Fishes of the word. 3rd edn. John Wiley and Sons Inc., New York. Oyhenart-Perera MF, Luengo JA and Brum-Zorrilla N (1975). Estudio citogenetico em Cichlasoma facetum (JEMNYNS) y Crenicichla sexatilis (LINN) (Teleostei, Cichlidae). Rev. Biol. Urug. 3: 29-36. Portela-Castro ALB (1999). Citogenética de peixes da subfamília tetragonopterinae (Pisces, Characidae), Aspectos citotaxonômicos e Evolutivos. Doctoral thesis, NUPELIA, Universidade Estadual de Maringá, Maringá. Renesto E, Zawadzki CH and Revaldaves E (2001). Biochemical taxonomy of Crenichla (Pisces, perciformes, Cichlidae) of the Iguaçu River, Brazil. Braz. Arch. Biol. Technol. 44: 15-22. Roncati HL, Fenocchio AS, Pastori MC, Sanches S, et al. (1996). Descripcion cariotipica de cinco gêneros de ciclideos (Perciformes) de la Republica Argentina. In: VI Simpósio de Citogenética Evolutiva e Aplicada de Peixes Neotropicais, São Carlos, SP, Brazil, 90. Salgado SM, Feldberg E and Porto JIR (1994). Estudos citogenéticos na família Cichlidae (Perciformes, Labroidei) da bacia amazônica central. In: VII Simpósio de Citogenética Evolutiva e Aplicada de peixes Neotropicais, Botucatu, 47. Schweizer D (1976). Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosoma 58: 307-324. Sumner AT (1972). A simple technique for demonstrating centromeric heterochromatin. Exp. Cell Res. 75: 304-306. Thompson KW (1979). Cytotaxonomy of 41 species of Neotropical Cichlidae. Copeia 1979: 679-691. Wasko AP, Vênere PC and Galetti PM Jr (1996). Chromosome divergence between two sympatric characid fishes of the genus Bryconamericus. Braz. J. Genet. 19: 225-230. |
|