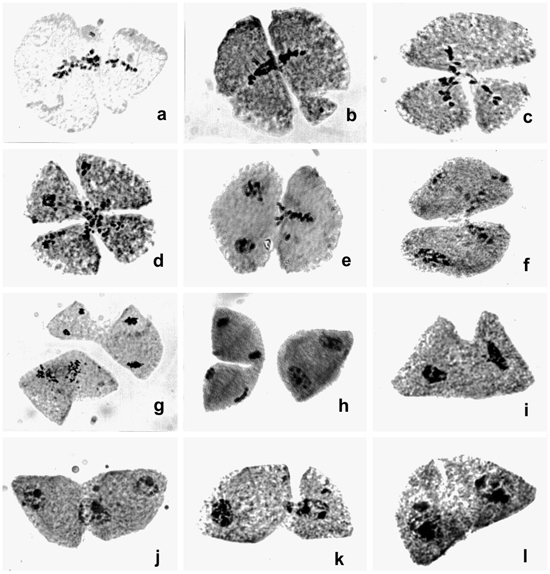
The accession of B. humidicola has 2n = 54 chromosomes. Based on the meiotic behavior in normal meiocytes, we suggest that it is a polyploid accession (2n = 9x = 54), derived from x = 6. Chromosome number counts in the B. humidicola collection at Embrapa Beef Cattle Center revealed accessions with 2n = 36, 42, and 54 chromosomes (Boldrini KR, unpublished data). Among the accessions with 2n = 54 chromosomes, another accession (H022) also presented an abnormal pattern of cytokinesis (Calisto V, unpublished data) similar to the abnormality reported in H121. However, in H022 the abnormal cytokinesis occurred only in meiocytes in which chromosome associations were disrupted at the end of diakinesis, separating the chromosomes by desynapsis; the 54 univalents were aligned forming a wide metaphase plate. In H121, normal cytokinesis after telophase I was also absent and the second cytokinesis after telophase II occurred normally. Cytological evidence of precocious cytokinesis was not found in the H121.
In another accession of B. humidicola (H003) with 2n = 7x = 42, also derived from x = 6, another abnormal pattern of cytokinesis was recorded (Boldrini et al., 2006). In this accession, the first cytokinesis occurred after telophase II, generating dyads with binucleated microspores, which initiated the second cytokinesis by invagination, giving rise to four normal microspores, after release from the callose wall. In H121 and in H022 (Calisto V, unpublished data), both with 2n = 54 chromosomes, cytokinesis was initiated marginally in metaphase I and migrated to the center, similar to an invagination process. Incomplete cytokinesis leading to 2n gamete formation was recorded in another accession of B. humidicola (H047), 2n = 36 chromosomes (Gallo et al., 2007). In this accession, the first cytokinesis was initiated in the center of the cell after telophase I but did not get to the borders, allowing rejoining of the nucleus after prophase II. The number of microsporocytes affected by abnormal cytokinesis recorded in these accessions of B. humidicola was always small. In accession H022, with a similar abnormality, 16.8% of the cells were affected.
A large number of genes, generally dominant, which are stage, site- and time-specific are involved in the control of meiosis (Gottschalk and Kaul, 1974, 1980a,b; Baker et al., 1976; Golubovskaya, 1979, 1989). Among genes acting in the meiotic process, those responsible for the partitioning of the cytoplasm after nuclear division play an important role in the formation of viable gametes. The timing of cytokinesis varies among angiosperms. In most monocotyledons, cytokinesis is successive, i.e, one partitioning of the cytoplasm occurs after telophase I and another after telophase II, so that there is a distinct dyad stage. However, in most dicots it is simultaneous and occurs after telophase II (Peirson et al., 1996). In higher plants, cytokinesis during mitosis is a genetically controlled multistep process. At least three cellular components play important roles in this process: i) the Golgi apparatus produces secretory vesicles and synthesizes the cell wall polysaccharides; ii) Golgi-derived vesicles fuse to form a cell plate, and iii) the cytoskeleton required for phragmoplast formation and expansion controls the cell division planes. Other cellular components, including the endoplasmic reticulum, intermediate filaments, calmodulin, and myosin may also play important roles in cytokinesis (Staehelin and Hepler, 1996). During mitosis in higher plants, a cortical ring of microtubules, called ‘the preprophase band’, marks the site where the cell plate will be formed, determining the division pattern. Meiosis, on the other hand, lacks the preprophase band, although the division pattern seems to be accurately controlled. Cytokinesis-defective mutants have been characterized in several species of higher plants during mitosis and meiosis (Beadle, 1932; Peirson et al., 1996; Hülskamp et al., 1997; Nickle and Meinke, 1998; Boldrini et al., 2006).
Absence of cytokinesis has been recorded for other Brachiaria species (Risso-Pascotto et al., 2003a; Utsunomiya et al., 2005). In these cases, the failure of cytokinesis occurred after telophase I or telophase II, generating balanced 2n gametes. In H121 and H022, cytokinesis occurred precociously during metaphase I. We suggest that the genetic control of cytokinesis in these meiocytes is activated very early and is not synchronized with karyokinesis, generating unbalanced gametes. Based on the cytological analysis of the B. humidicola collection, we suggest that this species has a greater tendency for abnormal cytokinesis than do other previously analyzed Brachiaria species (Mendes-Bonato et al., 2002, 2006a; Utsunomiya et al., 2005). Several putative meiotic mutations have been described in the genus Brachiaria (Mendes-Bonato et al., 2001, 2003, 2004, 2006b; Risso-Pascotto et al., 2002, 2003a, 2005; Mendes-Vieira et al., 2005) and also in the post-meiotic process (Junqueira Filho et al., 2003; Mendes-Bonato et al., 2004) which could represent putative mutations, suggesting that these genes were incorporated in the gene pool of the genus during its evolutionary process.
Some promising apomictic accessions of B. humidicola are under careful agronomic and grazing evaluation in hopes of selecting new cultivars. This species is well adapted to poorly drained and infertile acid soils (Keller-Grein et al., 1996), for which very few options are available, thus the urgent demand for improved cultivars. The occurrence of precocious cytokinesis detected in this accession affects pollen viability and unbalanced gametes are generated by partitioning of the genome in metaphase I. Other accessions of this species may be better progenitors in intra- and interspecific hybridization as pollen donors. However, the frequency of meiocytes affected by precocious cytokinesis in H121 (about of 10%) may not be enough to require that this accession be discarded from the breeding program. Other abnormalities due to polyploidy (2n = 9x = 54) were also detected: precocious chromosome migration to the poles in metaphase I (55.7%) and metaphase II (55.6%), and laggards in anaphase I (82.5%) and anaphase II (58.7%), which generated micronuclei in telophase I (44.9%) and II (11.4%).
Polyploidy in Brachiaria is correlated with apomixis, which bypasses meiosis in the megagametogenesis process. The embryo-sac is formed by parthenogenesis of a somatic cell, thus the embryo is maternal. But for seed development, the secondary nuclei of the embryo sac need to be fertilized by a male gamete - pseudogamy. Accessions with a high frequency of meiotic abnormalities due to polyploidy, which severely impair pollen viability, need to be discarded early in the breeding program to avoid passing on defective genes to the progenies. Until now the on-going hybridization program in Brachiaria has involved intra- and interspecific crosses only between tetraploid (2n = 4x = 36) genotypes derived from x = 9. The 2n = 54 chromosome pool is still impervious to breeding due to a lack of compatible sexual source for crossing. Interploid crosses have been unsuccessful in this genus (Hacker, 1988). Therefore, accession H121 cannot be used in crosses not only due to its high level of ploidy, but also due to its abnormal cytokinesis and differences in its basic chromosome number (x = 6).
ACKNOWLEDGMENTS
The authors are grateful to UNIPASTO for financial support.
REFERENCES
Baker BS, Carpenter AT, Esposito MS, Esposito RE, et al. (1976). The genetic control of meiosis. Annu. Rev. Genet. 10: 53-134.
Beadle GW (1932). A gene in Zea mays for failure of cytokineses during meiosis. Cytologia 3: 112-133.
Boldrini KR, Pagliarini MS and do Valle CB (2006). Abnormal timing of cytokinesis in microsporogenesis in Brachiaria humidicola (Poaceae: Paniceae). J. Genet. 85: 225-228.
Gallo PH, Micheletti PL, Boldrini KR, Risso-Pascotto C, et al. (2007). 2n gamete formation in Brachiaria (Poaceae: Paniceae). Euphytica 154: 255-260.
Golubovskaya IN (1979). Genetic control of meiosis. Int. Rev. Cytol. 58: 247-290.
Golubovskaya IN (1989). Meiosis in maize: mei genes and conception of genetic control of meiosis. Adv. Genet. 26: 149-192.
Gottschalk W and Kaul MLH (1974). The genetic control of microsporogenesis in higher plants. Nucleus 17: 133-166.
Gottschalk W and Kaul MLH (1980a). Asynapsis and desynapsis in flowering plants. I. Asynapsis. Nucleus 23: 1-15.
Gottschalk W and Kaul MLH (1980b). Asynapsis and desynapsis in flowering plants. II. Desynapsis. Nucleus 23: 97-120.
Hacker JB (1988). Sexuality and hybridization in signalgrass, Brachiaria decumbens. Trop. Grassl. 22: 139-144.
Hülskamp M, Parekh NS, Grini P, Schneitz K, et al. (1997). The STUD gene is required for male-specific cytokinesis after telophase II of meiosis in Arabidopsis thaliana. Dev. Biol. 187: 114-124.
Junqueira Filho RG, Mendes-Bonato AB, Pagliarini MS, Bione NC, et al. (2003). Absence of microspore polarity, symmetric divisions and pollen cell fate in Brachiaria decumbens (Gramineae). Genome 46: 83-88.
Keller-Grein G, Maass BL and Hanson J (1996). Natural variation in Brachiaria and existing germplasma collections. In: Brachiaria: biology, agronomy, and improvement (Miles JW, Maass BL and Valle CB, eds.). Centro Internacional de Agricultura Tropical, Colombia, 17-42.
Mendes-Bonato AB, Pagliarini MS, Valle CB and Penteado MIO (2001). A severe case of chromosome stickiness in pollen mother cells of Brachiaria brizantha (Hochst) Stapf (Gramineae). Cytologia 66: 287-291.
Mendes-Bonato AB, Pagliarini MS, Forli F, Valle CB, et al. (2002). Chromosome number and microsporogenesis in Brachiaria brizantha (Gramineae). Euphytica 125: 419-425.
Mendes-Bonato AB, Risso-Pascotto C, Pagliarini MS and Valle CB (2003). Normal microspore production after cell fusion in Brachiaria jubata (Gramineae). Genet. Mol. Biol. 26: 517-520.
Mendes-Bonato AB, Pagliarini MS, do Valle CB and Jank L (2004). Abnormal pollen mitoses (PM I and PM II) in an interspecific hybrid of Brachiaria ruziziensis and Brachiaria decumbens (Gramineae). J. Genet. 83: 279-283.
Mendes-Bonato AB, Pagliarini MS, Risso-Pascotto C and Valle CB (2006a). Chromosome number and meiotic behaviour in Brachiaria jubata (Gramineae). J. Genet. 85: 83-87.
Mendes-Bonato AB, Pagliarini MS and Valle CB (2006b). Abnormal spindle orientation during microsporogenesis in an interspecific Brachiaria (Gramineae) hybrid. Genet. Mol. Biol. 29: 122-125.
Mendes-Vieira D, Mendes-Bonato AB, Pagliarini MS and Valle CB (2005). Abnormal meiotic behavior in Brachiaria brizantha (Poaceae) leading to microspore degeneration. Caryologia 58: 396-402.
Nickle TC and Meinke DW (1998). A cytokinesis-defective mutant of Arabidopsis (cyt1) characterized by embryonic lethality, incomplete cell walls, and excessive callose accumulation. Plant J. 15: 321-332.
Peirson BN, Owen HA, Feldmann KA and Makaroff CA (1996). Characterization of three male-sterile mutants of Arabidopsis thaliana exhibiting alterations in meiosis. Sex. Plant Reprod. 9: 1-16.
Risso-Pascotto C, Pagliarini MS and Valle CB (2002). Abnormal nucleolar cycle in microsporogenesis of Brachiaria decumbens (Gramineae). Cytologia 67: 355-360.
Risso-Pascotto C, Pagliarini MS, Valle CB and Mendes-Bonato AB (2003a). Chromosome number and microsporogenesis in pentaploid accession of Brachiaria brizantha (Gramineae). Plant Breed. 122: 136-140.
Risso-Pascotto C, Pagliarini MS and Valle CB (2003b). A mutation in the spindle checkpoint arresting meiosis II in Brachiaria ruziziensis. Genome 46: 724-728.
Risso-Pascotto C, Pagliarini MS, Valle CB and Jank L (2005). Symmetric pollen mitosis I and suppression of pollen mitosis II prevent pollen development in Brachiaria jubata (Gramineae). Braz. J. Med. Biol. Res. 38: 1603-1608.
Staehelin LA and Hepler PK (1996). Cytokinesis in higher plants. Cell 84: 821-824.
Staiger CJ and Cande WZ (1990). Microtubule distribution in dv, a maize meiotic mutant defective in the prophase to metaphase transition. Dev. Biol. 138: 231-242.
Staiger CJ and Cande WZ (1991). Microfilament distribution in maize meiotic mutants correlates with microtubule organization. Plant Cell 3: 637-644.
Utsunomiya KS, Pagliarini MS and do Valle CB (2005). Microsporogenesis in tetraploid accessions of Brachiaria nigropedata (Ficalho & Hiern) Stapf (Gramineae). Biocell 29: 295-301.