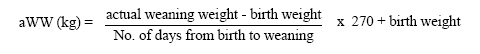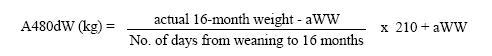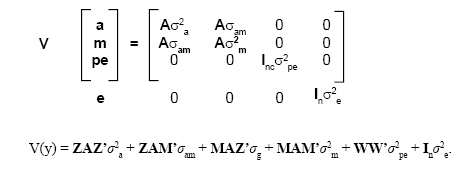|
Estimation of genetic parameters and variance components for growth traits in Romosinuano cattle in the Colombian humid tropics
R.M. Sarmiento1 and J.P. Garcia2
1Animal Genetics Resource Group, Tibaitatá Research Center,
Corporación Colombiana de Investigación Agropecuria CORPOICA,
Bogotá, Colombia
2Animal Genetics Resource Group, Turipaná Research Center, Córdoba,
Corporación Colombiana de Investigación Agropecuria CORPOICA, Montería, Colombia
Corresponding author: R.M. Sarmiento
E-mail: [email protected]
Genet. Mol. Res. 6 (3): 482-491 (2007)
Received January 10, 2007
Accepted May 11, 2007
Published August 7, 2007
ABSTRACT. (Co)variance components and genetic parameters were estimated for body weights of a Romosinuano herd located in Sinú Valley, Cordoba, Colombia. Restricted maximum likelihood methods were used with a univariate animal model for birth weight, weaning weight (270 days), 16-month weight (480 days), weaning daily gain, and post-weaning daily gain. Models included random animal direct and maternal genetic effects, maternal permanent environmental effect (c2), and sex-year-month of birth and age of dam, as fixed effects. Estimates of direct effect for birth weight, weaning weight, 480-day weight, weaning daily gain, and post-weaning daily gain were: 0.25 ± 0.0001, 0.34 ± 0.063, 0.33 ± 0.066, 0.32 ± 0.062, and 0.17 ± 0.052, respectively. Estimates of direct maternal genetic effects were low and ranged from 0.06 ± 0.003 for birth weight to 0.20 ± 0.054 for weaning daily gain. The genetic correlations between direct and maternal genetic effects were negative and low for 480-day weight (-0.05 ± 0.219) and showed values of -0.37 ± 0.007, -0.34 ± 0.133, -0.33 ± 0.135, and -0.38 ± 0.232 for birth, weaning weight, weaning, and post-weaning daily gain, respectively. Permanent environmental maternal effects were not significant; the highest values were found for weaning weight, and weaning daily gain (0.086 ± 0.031 and 0.078 ± 0.031, respectively). We conclude that direct and maternal effects should be included in a selection program for all of these traits, and also that selection of weaning weights would be the most productive way to improve performance in Romosinuano cattle.
Key words: Heritability, Genetic, Correlation, Growth
INTRODUCTION
The cattle herds in Colombia were initially composed of populations established by the Spanish in the 16th century. The descendants of these cattle are well adapted to hostile humid tropical conditions; however, during the second half of the past century these cattle were indiscriminately crossbred with the Zebu breed. That and the introduction of genetic material from temperate countries have been the main reasons for erosion of the genetic resources of local tropical cattle (Hall and Ruane, 1993). These cattle possess genetic properties that are essential for survival and reproduction in the tropics. It is important to preserve them for future use (Syrstad and Ruane, 1998).
Genetic improvement by selection is an attractive alternative for the introduction of foreign genetic material. Most populations of tropical cattle have been subjected to only little, if any, selection for beef production. The impressive results achieved by selection in many temperate breeds suggest that there should be good prospects for improving the potential of tropical cattle by the same method.
The Romosinuano was developed in the Sinú Valley of northern Colombia. They are of the Criollo type, red-brown and polled. The name Romosinuano means Polled Sinú. The Romosinuano originated during the late 1800s from Costeño con Cuernos (Horned Sinú). It is unknown whether the polled trait is an independent mutation within the Costeño con Cuernos or if Angus or Red Poll blood was introduced. The breed is somewhat smaller than the Costeño con Cuernos, with mature females weighing 400 kg and males 500 kg. The breed is docile and has typical beef conformation (Mason, 1996).
Romosinuanos are reported to be highly fertile and are noted for their longevity, docile temperament, and their combining ability with Bos indicus (de Alba, 1987; Derr et al., 1995; Martínez-Correal, 1998). Some of these considerations have been reported by Hernández-Ceron et al. (2004), who found that embryos of Brahman and Romosinuano breeds are more resistant to elevated temperature than Angus embryos. They concluded that the process of adaptation of Brahman and Romosinuano breeds to hot environments resulted in selection of genes controlling thermotolerance at the cellular level.
Chase Jr. et al. (1997) found that the body weight of Romosinuano bulls was lower than that of Brahman cattle and lower than Angus from weaning until about 12 months of age. By 12 months of age, body weights were similar for Romosinuano and Brahman bulls; this trend continued through 20 months of age. In the same report, the scrotal circumferences of Romosinuano and Angus bulls were similar throughout most of the study, and both were larger than those of Bos indicus bulls, with the largest differences observed from weaning through 17 months of age. These differences in scrotal circumference may be due to the fact that the Bos taurus bulls had reached puberty by 17 months of age.
Chase Jr. et al. (1997) reported that the average daily gain during winter for Romosinuano heifers born in 1991 was greater (P < 0.05) than that of Brahman heifers, but less (P < 0.05) than that of Angus and Hereford heifers (temperate Bos taurus breeds). In summer, the average daily gain of Romosinuano heifers was greater (P < 0.05) than for Angus and Hereford heifers, and similar to that of Brahman heifers.
Based on expected progeny differences, Elzo et al. (1998) showed that Romosinuano animals competed well against Zebu and Romosinuano-Zebu crossbred animals under tropical environmental conditions. We examined (co)variance components and genetic parameters of growth traits Romosinuano breed cattle raised in Sinú Valley Colombia.
MATERIAL AND METHODS
We examined data from a germplasm herd housed at the Turipaná Research Center, which is located in the Sinú Valley, Department of Córdoba, Colombia. This property is 13 m above sea level, has a mean annual temperature of 27°C, an annual precipitation of 1,200 mm, and a mean annual relative humidity of 82%. Cows were bred from April to June, and calving occurred between January and March. The original data file was checked and corrected for erroneous information.
The adjusted weight at weaning (aWW; 270 days) was:
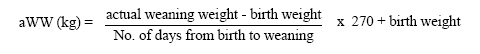
Adjusted 16-month weight (480 days; A480dW):
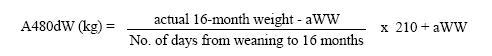
The data consisted of records of 4901 animals, the progeny of 191 sires and 1363 dams raised under Colombian humid tropical pasture conditions. Records were collected from 1980 to 2004. More than 2000 calf records were available for each of the four growth traits (Table 1).
Animals were arranged in contemporary groups, based on year, season, sex, and parity number. The general linear model procedure of SAS (1989) was used to test the significance of fixed effects and to test the models. A covariate for age of dam was included in the model for all traits. Random effects considered in the model were direct and maternal effects and permanent environmental effect of dams. The model in the analysis was a univariate animal model as follows:
Y = Xb + Za + Mm + Wpe + e
where Y = vector of observations, β = vector of fixed effects (year of birth, season, sex, and covariate for age of dam), a = vector of random additive direct genetic effects, m = vector of random maternal genetic effect, p = vector of random permanent environmental effects of dams, e = vector of random residual effects, and X, Z, M, and W are known incidence matrices relating records to the respective fixed and random effects. The first and the second moments of the model were assumed to be:
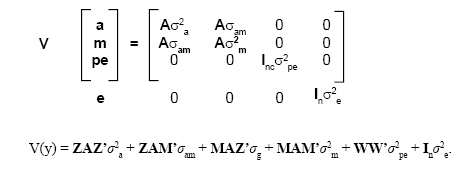
where σ2a is the direct additive genetic variance; σ2m and σ2pe are the maternal additive genetic and permanent environmental variances; σam is the covariance between additive direct and additive maternal genetic effects; σg is the genetic variance; σ2e is the residual variance, in this case, σ2te, temporary environmental variance; Inc and In are identity matrices of order equal to the number of dams (nc) and to the total number of animals with records (n), respectively, and A is the additive numerator relationship matrix of order q. The inverse of A was calculated using the rules of Quaas (1976), including ancestors of animals without records (Henderson, 1975).
Estimation of (co)variance components
The starting values of variance and covariance components and genetic parameters were initially estimated using a derivative-free restricted maximum likelihood procedure (DFREML). The last values of the variance components were estimated with derivative-free REML (Graser et al., 1987; Meyer, 1989) using multiple-trait derivative-free restricted maximum likelihood (MTDFREML) programs (Boldman et al., 1995).
The method involves maximizing the likelihood function (Λ) given the data and is the same as maximizing log Λ or minimizing -2 log Λ. Iterations were stopped when the variance of function values (-2 log Λ) in the simplex was less than 1 x 10-8. Each analysis was then restarted using the resulting estimates of the parameters as new priors until changes in the function value and estimates of the scaled parameters (variances as proportions of phenotypic variance and correlations) were less than 0.01 (Boldman et al., 1995).
RESULTS
The data structure is summarized in Table 1. Though only a single herd was included, the number of sires and dams with progeny records was over 150 and 1000, respectively, although this was higher for birth weight and lower for post-weaning daily gain due to losses from several causes (death, sale, etc.) but not because of selection, because this population belongs to an in situ conservation program in which the sires have been selected for minimum kinship.
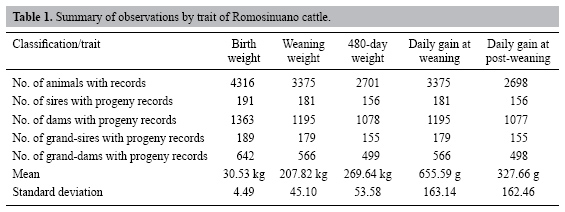
The direct additive genetic variances were larger than maternal genetic variances for all traits (Table 2). Larger values of direct and maternal variance were found for daily gain pre- and post-weaning. Genetic covariances between direct and maternal genetic effects were negative for all traits. The lowest value for permanent environmental variance was found for birth weight and the highest error variance was observed for post-weaning daily gain.
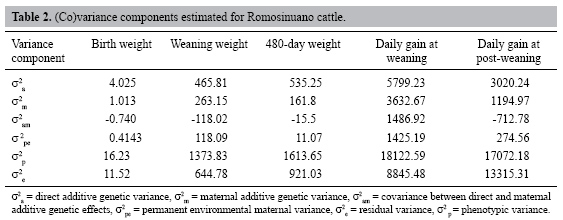
Estimates of heritability and correlations between direct and maternal effects were calculated (Table 3). Direct heritability was moderate for all traits except post-weaning daily gain, which had the lowest value. The maternal heritability gave very low values and was lower than direct heritability for all traits; the highest value was found for weaning daily gain and the lowest value was found for birth weight. The highest values for total heritability were found for weaning weight and weaning daily gain and the lowest value was found for post-weaning daily gain.
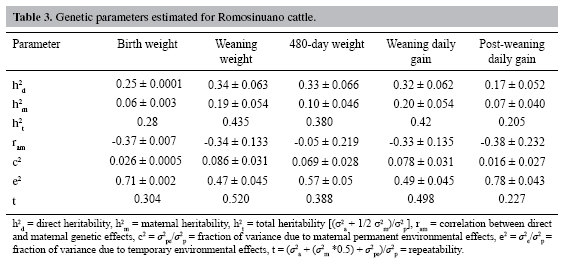
Estimates of direct-maternal genetic correlation were moderately large and negative for all traits except for 480-day weight, which gave the lowest value (Table 3) and the highest standard deviation. The proportion of variance due to permanent environmental effects was small, being lowest for post-weaning daily gain and highest for weaning weight.
DISCUSSION
The direct additive genetic variance was larger than the maternal additive genetic variance, which was also observed by Lee et al. (2000) in Native Korean cattle, Meyer et al. (1993) in Australian beef cattle, using a multibreed model, Koch et al. (1994) in Hereford cattle, and Aziz et al. (2005) in Japanese Black cattle, using random regression models. However, Elzo et al. (1998), in a study of Romosinuano-Zebu multibreed data, observed that the additive genetic variance was lower than maternal additive genetic variance in Romosinuano cattle for birth weight and post-weaning weight (2.04, 100.46 for direct and 2.32, 166.52 for maternal) and observed similar values for weaning weight (0.09). However, the Zebu breed had higher values for direct additive genetic variance than for maternal additive genetic variance in the same traits (3.68, 155.5 for direct and 2.23, 74.83 for maternal additive genetic variance).
Estimates of direct and maternal heritability for all traits in the Romosinuano cattle were within the range of values reported for Bos taurus breeds. The direct heritability that we observed was lower than those found by Iwaisaki et al. (2005) in Gelbvieh cattle; they reported estimates of direct heritabilities for birth (0.52 ± 0.05 and 0.51 ± 0.07), weaning (0.36 ± 0.05 and 0.28 ± 0.06) and yearling weight (0.59 ± 0.15 and 0.48 ± 0.08) using both multitrait and random regression models, respectively. Koch et al. (1994) in Hereford cattle, found higher values for birth weight (0.46), post-weaning daily gain (0.41) and post-weaning weight (0.42), but lower values for weaning weight (0.15) and weaning daily gain (0.17).
Direct heritability estimates were moderately high for weaning weight, 480-day weight and weaning daily gain, and were somewhat higher than reported by Elzo and Wakeman (1998) in an Angus-Brahman multibreed herd for birth weight (0.22 and 0.23) and weaning weight (0.25 and 0.29), and also by Elzo et al. (1998) in a Romosinuano-Zebu multibreed herd for birth weight, weaning weight and post-weaning daily gain (Romosinuano: 0.16, 0.09, 0.14; Zebu: 0.24, 0.10, 0.14 for each trait, respectively), and the estimates of heritability for direct genetic effects in the Romosinuano-Zebu multibreed herd tended to be smaller than our values for all traits (0.21, 0.05, and 0.12, respectively). The environmental factors might have had a smaller effect on our populations during the last eight years, as this was the same Romosinuano population used by Elzo et al. (1998); however, some herd conditions could have changed. Changes in pastures, weaning handling, mating seasons, etc., could have diminished the environmental variance.
Covariance functions for growth since birth to 630 days have been estimated in Nelore cattle (Albuquerque and Meyer, 2001); lower values for direct effect (heritability) after birth displayed a trend to be lowest when the maternal effect was highest. The maternal heritability estimates were found to be higher at 110 to 120 days of age, diminishing later. But in our study, lower values were found for birth weight and post-weaning, and higher values at weaning, both for direct and maternal additive genetic effects.
Maternal heritabilities were lower than direct heritabilities for all traits. These effects do not seem very important for birth weight, 480-day weight and post-weaning daily weight. Variance components for maternal effects for these traits were less than 10% of phenotypic variance. However, the maternal effects for weaning weight and weaning daily gain were more than 19% of phenotypic variance. But in animals raised on pasture and with only mineral supplementation, as in our herd, the length of time from weaning to 18 months may be enough for compensatory gain to maintain some of the maternal effects that exist at weaning, as was described by Eler et al. (1995).
Similar results were found by Plasse et al. (2002) for a Brahman herd, who reported estimates of maternal heritability for birth and 480-day weight of 0.07 and 0.04, respectively. Ferreira et al. (1999) reported similar values in a Hereford herd for birth weight (0.08) and weaning weight (0.17). Higher values were reported by Elzo et al. (1998) in Romosinuano (0.18, 0.23) and Zebu breeds (0.14, 0.23), for birth weight and post-weaning daily gain, respectively, but lower values for weaning weight (0.09 in both cases). Also, Elzo and Wakeman (1998) in a study of Angus (0.17, 0.18) and Brahman herds (0.18, 0.21) reported higher values for birth and weaning weight, respectively, possibly because they used multibreed models.
For Elzo et al. (1998), the values of maternal heritabilities for weaning daily gain in Romosinuano (0.23) and Zebu (0.07) suggest that the pre-weaning maternal environment continues to affect calf growth after weaning and that this post-weaning maternal effect is more important for the progeny of Romosinuano than the progeny of Zebu dams. Similarly, our results suggest an important maternal pre-weaning effect; however, there was a less important effect for post-weaning (0.07) and 480-day weight (0.1). Apparently the post-weaning growth has little influence on milk performance of the dam, possibly due to changes in pasture conditions without supplements, which severely diminished the growth trend, and therefore at a later age, the weight depends principally on individual performance.
A negative genetic (co)variance between direct and maternal genetic effects has also been observed by Wright et al. (1991), Ferreira et al. (1999), Dodenhoff et al. (1999), Norris et al. (2004), and Iwaisaki et al. (2005). However, other studies found the covariance between direct and maternal genetic effects to be positive for weaning weight and post-weaning daily gain (Plasse et al., 2002) but negative for birth weight in Romosinuano cattle and negative for all three traits in Zebu cattle (Elzo et al., 1998), similar what we observed.
The correlation between direct and maternal effects was low and negative for 480-day weight (-0.05 ± 0.21) and moderate for birth weight, weaning weight and pre- and post-weaning daily gain (-0.37 ± 0.007, -0.34 ± 0.133, -0.33 ± 0.135, -0.38 ± 0.232, respectively), the signs of these estimates of the direct and maternal correlation agree with what was found by Meyer (1992b), who reported for yearling weight an estimate of -0.48 in Australian Hereford cattle and Diop (1997) who reported an estimate of -0.50 in Gobra cattle in Senegal. The negative correlation between direct and maternal genetic effects could be an indication of genetic antagonism between genes (Ferraz et al., 2000), and it may, therefore, be important to consider the genetic correlation in selection programs.
In Romosinuano cattle, Elzo et al. (1998) have reported positive genetic correlations between direct and maternal effects for weaning weight (0.23) and post-weaning daily gain (0.08) but negative for birth weight (-0.19). However, the Zebu cattle in the same report showed a low and positive value only for post-weaning weight (0.02) and negative for birth weight and weaning weight (-0.18 and -0.50, respectively).
The maternal permanent environmental effects were lower for birth weight and post-weaning daily gain than for weaning weight, weaning daily gain and 480-day weight. Higher values were reported by Azis et al. (2005), who found values ranging from 0.12 to 0.40 for weight during 90 to 270 days, with a linear upward trend. Similar to our results, Lee et al. (2000) found values close to 0.0 for maternal permanent environmental effect on yearling weight (ranging between 0.0 and 0.03), 18-month weight (0.01) and slaughter weight (0.0), using several models. Meyer et al. (1993) found that maternal permanent environmental effects vary according to the model used, reporting higher values (0.17, 0.31, and 0.13 for birth, weaning and yearling weight, respectively) when the model did not include maternal genetic effects; but when the model included these effects (with or without interaction between direct and maternal genetic effects), a dramatic decrease (0.09, 0.20 and 0.05) was observed. We used a model with direct, maternal and interaction between effects, expecting that this model would reduce the maternal environmental effects.
For weaning weight and weaning daily gain, although they gave higher values, the maternal environmental effects (c2) explained the low variation between animals as genetic differences in growth potential. The low estimate for c2 of 8.7%% was in disagreement with previous results for Hereford breeds (polled and horned), ranging from 21 to 34% (Boldman et al., 1991; Meyer, 1992a; Waldron et al., 1993). This reinforces the evidence for clear differences between breeds in the contribution of the different components of dams’ maternal effects on the growth of their calves, as was proposed by Meyer et al. (1993).
Direct heritability estimates of weights taken at different ages were moderately high, whereas those due to maternal effects were low, but significant. The largest direct and maternal heritabilities were for weaning weight at 120 days of age and weaning daily gain, indicating that genetic improvement of these traits can be obtained through selection in this Romosinuano herd. Genetic correlations between direct and maternal genetic effects for each trait were negative and moderate. It is suggested that pre-weaning growth could be used to select for weights at later ages, which will have a positive effect on weights at later ages. Further studies on mothering ability and milk production of dams are warranted.
ACKNOWLEDGMENTS
The authors thank Leonardo Alvarado and Jorge Garcés for the supervision of data collection and processing at the Turipaná Research Center. The analysis of these data was supported by the Ministerio de Agricultura y Desarrollo Rural and the Instituto Colombiano Agropecuario through the Project: Bancos de germoplasma animal.
REFERENCES
Albuquerque LG and Meyer K (2001). Estimates of covariance functions for growth from birth to 630 days of age in Nelore cattle. J. Anim. Sci. 79: 2776-2789.
Aziz MA, Nishida S, Suzuki K and Nishida A (2005). Estimation of direct and maternal genetic and permanent environmental effects for weights from birth to 356 days of age in a herd of Japanese Black cattle using random regression. J. Anim. Sci. 83: 519-530.
Boldman KG, Van Vleck LD and Gregory KE (1991). Estimates of direct and maternal parameters for 200d weight in purebred and composite lines of beef cattle. J. Anim. Sci. 69: 203.
Boldman KG, Kriese LA, Van Vleck LD, Van Tassell CP, et al. (1995). A manual for use of MTDFREML. A set of programs to obtain estimates of variances and covariances. ARS, USDA, Washington.
Chase CC Jr, Hammond AC, Olson TA, Murphy CN, et al. (1997). Introduction and evaluation of Romosinuano in the USA. Latin-Am. Arch. Anim. Prod. 5: 57-71.
de Alba J (1987). Criollo cattle of Latin America. In: Animal Genetic Resources: Strategies for Improved Use and Conservation. Animal Production and Health Paper (Hodges J, ed.). Food and Agriculture Organization (F.A.O.) of the United Nations, Rome, 19-66.
Derr JN, Davis SK, Estrada JL, Ossa JE, et al. (1995). Genetic characterization and conservation of Colombian criollo cattle. In: Conservation of domestic animal genetic resources (Crawford RD, Lister EE and Buckley JT, eds.). Rare Breeds International, Warwickshire, 307-313.
Diop M (1997). Design and analysis of open nucleus breeding systems for cattle in Senegal. Ph.D. thesis, University of Nebraska, Lincoln.
Dodenhoff J, van Vleck LD and Wilson DE (1999). Comparison of models to estimate genetic effects of weaning weight of Angus cattle. J. Anim. Sci. 77: 3176-3184.
Eler JP, van Vleck LD, Ferraz JB and Lobo RB (1995). Estimation of variances due to direct and maternal effects for growth traits of Nelore cattle. J. Anim. Sci. 73: 3253-3258.
Elzo MA and Wakeman DL (1998). Covariance components and prediction for additive and nonadditive preweaning growth genetic effects in an Angus-Brahman multibreed herd. J. Anim. Sci. 76: 1290-1302.
Elzo MA, Manrique C, Ossa G and Acosta O (1998). Additive and nonadditive genetic variability for growth traits in the Turipana Romosinuano-Zebu multibreed herd. J. Anim. Sci. 76: 1539-1549.
Ferraz JBS, Eler JP and Ribiero PMT (2000). Genetic study of Santa Gertrudis in Brazil. Livestock Research for Rural Development (http://www.cipav.org.co/Irrd/Irrd12/2/ferr122a.htm).
Ferreira GB, MacNeil MD and van Vleck LD (1999). Variance components and breeding values for growth traits from different statistical models. J. Anim. Sci. 77: 2641-2650.
Graser HU, Smith SP and Tier B (1987). A derivative-free approach for estimating variance components in animal models by restricted maximum likelihood. J. Anim. Sci. 64: 1362-1370.
Hall SJ and Ruane J (1993). Livestock breeds and their conservation: a global overview. Conserv. Biol. 7: 815-825.
Henderson CR (1975). Best linear unbiased prediction under a selection model. Biometrics 31: 423.
Hernandez-Ceron J, Chase CC Jr and Hansen PJ (2004). Differences in heat tolerance between preimplantation embryos from Brahman, Romosinuano, and Angus breeds. J. Dairy Sci. 87: 53-58.
Iwaisaki H, Tsuruta S, Misztal I and Bertrand JK (2005). Genetic parameters estimated with multitrait and linear spline-random regression models using Gelbvieh early growth data. J. Anim. Sci. 83: 757-763.
Koch RM, Cundiff LV and Gregory KE (1994). Cumulative selection and genetic change for weaning or yearling weight or for yearling weight plus muscle score in Hereford cattle. J. Anim. Sci. 72: 864-885.
Lee JW, Choi SB, Jung YH, Keown JF, et al. (2000). Parameter estimates for direct and maternal genetic effects on yearling, eighteen-month, and slaughter weights of Korean native cattle. J. Anim. Sci. 78: 1414-1421.
Martínez-Correal G (1998). El ganado criollo Romosinuano (Romo), animal genetic resources information. FAO 24: 1-11.
Mason IL (1996). A world dictionary of livestock breeds, types and varieties. 4th edn. CAB International, Wallingford.
Meyer K (1989). Restricted maximum likelihood to estimate variance components for animal models with several random effects using a derivative-free algorithm. Genet. Sel. Evol. 21: 317.
Meyer K (1992a). Variance components due to direct and maternal effects for growth traits of Australian beef cattle. Livest. Prod. Sci. 31: 179-204.
Meyer K (1992b). Bias and sampling covariances of estimates of variance components due to maternal effects. Genet. Sel. Evol. 24: 487-509.
Meyer K, Carrick MJ and Donnelly BJ (1993). Genetic parameters for growth traits of Australian beef cattle from a multibreed selection experiment. J. Anim Sci. 71: 2614-2622.
Norris D, Banga C, Benyi K and Sithole BC (2004). Estimation of genetic parameters and variance components for growth traits of Nguni cattle in Limpopo Province, South Africa. Trop. Anim. Health Prod. 36: 801-806.
Plasse D, Verde O, Arango J, Camaripano L, et al. (2002). (Co)variance components, genetic parameters and annual trends for calf weights in a Brahman herd kept on floodable savanna. Genet. Mol. Res. 1: 282-297.
Quaas RL (1976). Computing the diagonal element and inverse of a large numerator relationship matrix. Biometrics 32: 949-953.
SAS Institute Inc. (1989). SAS/STAT User’s guide. Version 6. 4th edn. Vol. 1. SAS Institute Inc., Cary.
Syrstad O and Ruane J (1998). Prospects and strategies for genetic improvement of the dairy potential of tropical cattle by selection. Trop. Anim. Health Prod. 30: 257-268.
Waldron DF, Morris CA, Baker RL and Johnson DL (1993). Maternal effects for growth traits in beef cattle. Livest. Prod. Sci. 35: 57-70.
Wright DW, Johnson ZB, Brown CJ and Wildeus S (1991). Variance and covariance estimates for weaning weight of Senepol cattle. J. Anim. Sci. 69: 3945-3951.
|