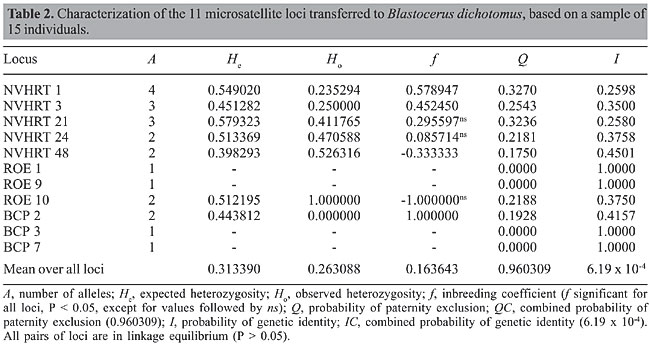
ABSTRACT. Blastocerus dichotomus, the marsh deer, is the largest Brazilian Cervidae species. The species is endangered because of hunting and loss of its natural habitat, i.e., flood plain areas, because of hydroelectric power station construction and agricultural land expansion. In the present study, we tested 38 microsatellite loci from four Cervidae species: Odocoileus virginianus (7), Rangifer tarandus (17), Capreolus capreolus (7), and Mazama bororo (7). Eleven loci showed clear amplification, opening a new perspective for the generation of fundamental population genetic data for devising conservation strategies for B. dichotomus. Key words: Microsatellites, Cervidae, Transferability, Marsh deer, Blastocerus dichotomus INTRODUCTION The marsh deer, Blastocerus dichotomus (Artiodactyla, Cervidae), is the largest deer of South America, reaching a size of up to 150 kg and 1.20 m high. The species occurs in flood plains from Southwest Peru, Paraguay, Bolivia, Northeast Argentina, and Northwest Uruguay, to the edges of the Amazon forest in Brazil (Mauro et al., 1998). Agricultural expansion and the construction of several hydroelectric power stations have been changing the original landscape of flood plains, reducing and isolating favorable habitats, which jeopardize the species viability (Tomas et al., 1997; Travassos, 2001). Because of that, the species is listed in the World Conservation Union (IUCN) Red List (IUCN, 2004). Transferability of microsatellite loci among closely related species is a consequence of the homology of the flanking regions of the simple-sequence repeats. Besides the possibility of comparative map construction among related species (Slate et al., 1998), transferability may reduce the cost of genotyping, opening new perspectives for the development of population genetic studies. The high rate of transferability has already been reported for plant species (e.g., Dayanan et al., 1997; White and Powell, 1997; Brondani et al., 1998; Collevatti et al., 1999) and among animal species, such as human and chimpanzee (Deka et al., 1994) and dog and fox (Fredholm and Wintero, 1995). The rate of transferability across Artiodactyla species is surprisingly high, even between different families, such as Cervidae and Bovidae, indicating a high genome homology (Engel et al., 1996; Talbot et al., 1996; Kühn et al., 1996; Roed and Midthjell, 1998; Roed, 1998; Slate et al., 1998; Broders et al., 1999; Cronin et al., 2003). We are interested in understanding the population genetic structure and gene flow among remnant populations of the marsh deer, Blastocerus dichotomus, to obtain useful information for the development of conservation strategies. In this study, we present the results of the transferability of microsatellite loci from four species of the Cervidae family to B. dichotomus: the caribou, Rangifer tarandus (Roed and Midthjell, 1998); the white-tailed deer, Odocoileus virginianus (DeWoody et al., 1995); the roe deer, Capreolus capreolus (Fickel and Reinsch, 2000), and the small red brocket deer, Mazama bororo (Duarte JMB, unpublished results). MATERIAL AND METHODS For the transferability analysis, a small sample of blood was collected in a vacutainer, from 15 individuals of B. dichotomus captured at a remnant area of a flood plain near the hydroelectric plant of Porto Primavera on the Paraná River (São Paulo, Brazil). DNA was extracted with the QIAamp Blood Kit (Quiagen, Hilden, The Netherlands), following the manufacturer’s instructions. For sampling, the animals were captured with darts and immobilized with the aid of ketamine chloride (9.56 mg/kg) and xylazine chloride (1.6 mg/kg) anesthesia, at doses prescribed to maintain the animal immobilized but conscious for 30-45 min. The individuals were handled following ASM guidelines. Thirty-eight microsatellite loci were tested (Table 1): 7 from O. virginianus; 17 from R. tarandus; 7 from C. capreolus, and 7 from M. bororo. For transferability analysis, each locus was first amplified using four individuals of B. dichotomus, randomly chosen from the 15 individuals sampled. PCR amplifications were performed in 13-µL reaction mix containing 0.9 µM of each primer, 1 unit Taq DNA polymerase (Phoneutria, Belo Horizonte, MG, Brazil), 200 µM of each dNTP, 1X reaction buffer (10 mM Tris-HCl, pH 8.3, 50 mM KCl, 1.5 mM MgCl2), 25 µg BSA, and 10.0 ng of template DNA. Amplifications were performed using a PE 9700 thermal controller (Applied Biosystems, MD) with the following conditions: 96°C for 2 min, 94°C for 1 min, 58° to 46°C for 1 min (according to each primer), 72°C for 1 min (30 cycles), and 72°C for 10 min. The amplified products were separated on 4% denaturing polyacrylamide gels stained with silver nitrate (Bassam et al., 1991) and sized by comparison to a 10-bp DNA ladder standard (Invitrogen, MD). 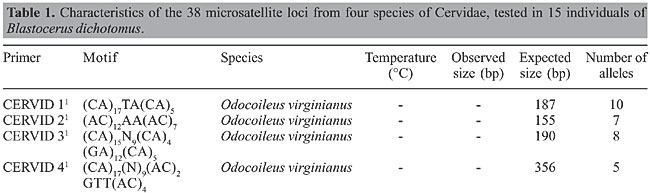       For those loci that showed clear and reproducible amplification, 15 individuals were genotyped for locus characterization. PCR amplification and polymorphism detection followed the same protocol and conditions described above. The number of alleles per locus, observed and expected heterozygosities under Hardy-Weinberg (Nei, 1978), and inbreeding coefficient (f) were estimated (Weir and Cockerham, 1984). Analyses were performed with FSTAT 2.9.3.2 (Goudet, 2002) and randomization based tests with Bonferroni correction were performed generating the log-likelihood statistic G to test for deviation from Hardy-Weinberg expectations and linkage disequilibrium (Goudet et al., 1996). Additionally, probability of genetic identity (I) (Chakravarati and Li, 1983), which corresponds to the probability of two random individuals displaying the same genotype, and paternity exclusion probability (Q) (Weir, 1996), which corresponds to the power with which a locus excludes an individual of being the parent of an offspring, were estimated. The combined probability of paternity exclusion, QC = 1 - [P (1 - Qi)] and the combined probability of genetic identity IC = P Ii were also estimated for the battery of loci. RESULTS AND DISCUSSION From the 38 primers tested in this study, 11 showed a robust amplification, with observed fragment sizes inside the expected range (Table 1). For loci NVHRT1, NVHRT3, NVHRT48, and BCP7, observed heterozygosity was lower than expected under Hardy-Weinberg equilibrium leading to a high and significant inbreeding coefficient (Table 2). The significant inbreeding and the low number of alleles detected in this study may be the outcome of sampling design, since all individuals were sampled in the same population and may be highly related. In the present study, we aimed to transfer a battery of heterologous loci to B. dichotomus. Further analysis will be carried out with several populations of B. dichotomus to study the genetic structure using the primers selected here. In Brazil, there are eight species of deer: Mazama americana, M. nana, M. gouazoubira, M. nemorivaga, M. bororo, Odocoileus virginianus cariacus, Blastocerus dichotomus, and Ozotoceros bezoarticus (Duarte, 1996; Rossi, 2000). This first report of the transferability of microsatellite loci to Brazilian Cervidae species opens a new perspective for the generation of fundamental population genetic data for devising conservation strategies for these species and for the construction of a comparative genetic map.
ACKNOWLEDGMENTS Research supported by CESP (Energetic Corporation of São Paulo, Brazil), whose assistance we gratefully acknowledge. REFERENCES Bassam BJ, Caetano-Anolles G and Gresshoff PM (1991). Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal. Biochem. 196: 80-83. Broders HG, Mahoney SP, Montevecchi WA and Davidson WS (1999). Population genetic structure and the effect of founder events on the genetic variability of moose, Alces alces, in Canada. Mol. Ecol. 8: 1309-1315. Brondani RPV, Brondani C, Tarchini R and Grattapaglia D (1998). Development, characterization and mapping of microsatellite markers in Eucalyptus grandis and E. uruphylla. Theor. Appl. Genet. 97: 816-827. Chakravarati A and Li CC (1983). The effect of linkage on paternity calculations. In: Inclusion probabilities in parentage testing (Walkera RH, ed.). American Association of Blood Banks, Arlington, 411-422. Collevatti RG, Brondani RV and Grattapaglia D (1999). Development and characterization of microsatellite markers for genetic analysis of a Brazilian endangered tree species Caryocar brasiliense. Heredity 83: 748-756. Cronin MA, Patton JC, Balmysheva N and MacNeil MD (2003). Genetic variation in caribou and reindeer (Rangifer tarandus). Anim. Genet. 34: 33-41. Dayanandan S, Bawa KS and Kesseli R (1997). Conservation of microsatellites among tropical trees (Leguminosae). Am. J. Bot. 84: 1658-1663. Deka R, Shriver MD, Yu LM, Jin L, et al. (1994). Conservation of human chromosome 13 polymorphic microsatellite (CA)n repeats in chimpanzees. Genomics 22: 226-230. DeWoody JA, Honeycutt RL and Skow LC (1995). Microsatellite markers in white-tailed deer. J. Hered. 86: 317-319. Duarte JMB (1996). Guia de identificação de cervídeos brasileiros. Funep, Jaboticabal. Engel SR, Linn RA, Taylor JF and Davis SK (1996). Conservation of microsatellite loci across species of Artiodactyls: implications for population studies. J. Mammal. 77: 504-518. Fickel J and Reinsch A (2000). Microsatellite markers for the European roe deer (Capreolus capreolus). Mol. Ecol. 9: 994-995. Fredholm M and Wintero AK (1995). Variation of short tandem repeats within and between species belonging to the Canidae family. Mamm. Genome 6: 11-18. Goudet J (2002). FSTAT: a program to estimate and test gene diversities and fixation indices.Version 2.9.3.2. Available at http://www.unil.ch/izea/softwares/fstat.html. Goudet J, Raymond M, de Meeus T and Rousset F (1996). Testing differentiation in diploid populations. Genetics 144: 1933-1940. IUCN (International Union for Conservation of Nature and Natural Resources) (2004). The IUCN mammal red data book. IUCN, Gland. Kühn R, Anastassiadis C and Pirchner F (1996). Transfer of bovine microsatellites to the cervine (Cervus elaphus). Anim. Genet. 27: 199-201. Mauro RA, Mourão GN, Coutinho ME, Silva MP, et al. (1998). Abundance and distribution of marsh deer Blastocerus dichotomus (Artiodactyla: Cervidae) in the Pantanal, Brazil. Rev. Ecol. Latinoam. 5: 13-20. Nei M (1978). Estimation of average heterozygosity and genetic distance from a small number of individual. Genetics 89: 583-590. Roed KH (1998). Microsatellite variation in Scandinavian Cervidae using primers derived from Bovidae. Hereditas 129: 19-25. Roed KH and Midthjell L (1998). Microsatellites in reindeer, Rangifer tarandus, and their use in other cervids. Mol. Ecol. 7: 1773-1776. Rossi RV (2000). Taxonomia de Mazama Rafinesque, do Brasil (Artiodactyla, Cervidae). MSc thesis, Instituto de Biociências, USP, São Paulo. Slate J, Coltman DW, Goodman SJ, MacLean I, et al. (1998). Bovine microsatellite loci are highly conserved in red deer (Cervus elaphus), sika deer (Cervus nippon) and Soay sheep (Ovis aries). Anim. Genet. 29: 307-315. Talbot J, Haigh J and Plante Y (1996). A parentage evaluation test in North American elk (Wapiti) using microsatellites of bovine and bovine origin. Anim. Genet. 27: 117-119. Tomas WM, Beccaceci MD and Pinder L (1997). Cervo-do-Pantanal (Blastocerus dichotomus). In: Biologia e conservação de cervídeos sul-americanos: Blastocerus, Ozotocerus e Mazama (Duarte JMB, ed.). FUNEP, Jaboticabal, 24-40. Travassos LEP (2001). Impactos gerados pela UHE Porto Primavera sobre o meio físico e biótico de Campinal, Presidente Epitácio, SP. Revista de Biologia e Ciências da Terra 1, Campina Grande. Weir BS (1996). Genetic data analysis II. Sinauer Associates, Sunderland. Weir BS and Cockerham CC (1984). Estimating F-statistics for the analysis of population structure. Evolution 38: 1358-1370. White G and Powell W (1997). Cross-species amplification of SSR loci in the Meliaceae family. Mol. Ecol. 6: 1195-1197. |
|