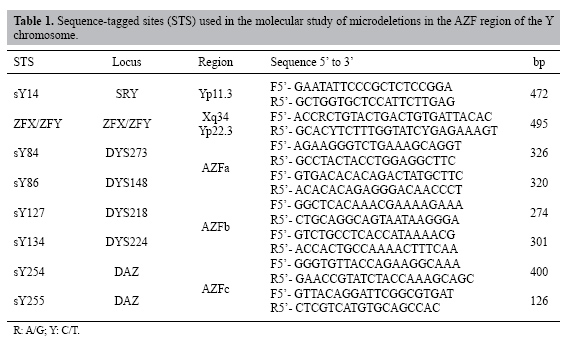
| Y chromosome microdeletions in Brazilian fertility clinic patients J.T. Arruda1, B.M. Bordin1, P.R. Santos1, W.E.J.C. Mesquita1, R.C.P.C. Silva2, M.C.S. Maia2, M.S. Approbato2, R.S. Florêncio2, W.N. Amaral2, M.A. Rocha Filho2 and K.K.V.O. Moura1,3 1Núcleo de Pesquisas Replicon, Universidade Católica de Goiás, Goiânia, GO, Brasil 2Hospital das Clínicas, Universidade Federal de Goiás, Goiânia, GO, Brasil 3Departamento de Biomedicina e Biologia, Universidade Católica de Goiás, Goiânia, GO, Brasil Corresponding author: J.T. Arruda E-mail: [email protected] Genet. Mol. Res. 6 (2): 461-469 (2007) Received December 22, 2006 Accepted June 05, 2007 Published June 30, 2007 ABSTRACT. Microdeletions in Yq are associated with defects in spermatogenesis, while those in the AZF region are considered critical for germ cell development. We examined microdeletions in the Y chromosomes of patients attended at the Laboratory of Human Reproduction of the Clinical Hospital of the Federal University of Goiás as part of a screening of patients who plan to undergo assisted reproduction. Analysis was made of the AZF region of the Y chromosome in men who had altered spermograms to detect possible microdeletions in Yq. Twenty-three patients with azoospermia and 40 with severe oligozoospermia were analyzed by PCR for the detection of six sequence-tagged sites: sY84 and sY86 for AZFa, sY127 and sY134 for AZFb, and sY254 and sY255 for AZFc. Microdeletions were detected in 28 patients, including 10 azoospermics and 18 severe oligozoospermics. The patients with azoospermia had 43.4% of their microdeletions in the AZFa region, 8.6% in the AZFb region and 17.4% in the AZFc region. In the severe oligozoospermics, 40% were in the AZFa region, 5% in the AZFb region and 5% in the AZFc region. We conclude that microdeletions can be the cause of idiopathic male infertility, supporting conclusions from previous studies. Key words: Male infertility, AZF, Assisted human reproduction, Y microdeletions INTRODUCTION Male infertility was first found to be associated with deletions on the Y chromosome by Tiepolo and Zuffardi (1976). They found that six men had loss of the distal euchromatic region of the Y chromosome (Yq11), while their fathers had the normal Y chromosome, characterizing these mutations as "de novo"; later they named this the AZF (azoospermia factor) locus. Based on molecular studies of infertile men who presented interstitial deletions (not detectable in the karyotype), it was found that this region is strongly associated with spermatogenesis defects and that it contains several genes involved in male fertility (Ferrás et al., 2004a; Rao et al., 2004). The microdeletions that occur in the AZF region affect genes that are involved in spermatogenesis (Dada et al., 2003). The AZF region is subdivided into three non-overlapping sub-regions called AZFa in the proximal portion (interval D3-D6), AZFb in the intermediate region (D13-D16) and AZFc in the distal region (D20-D22) (Foresta et al., 2001). Each of these regions contains several genes involved in male fertility that are in the euchromatic region of Yq; they are strongly associated with spermatogenic defects, such as azoospermia and oligozoospermia. Although there is not still no definitive consensus about the relationship between the type of microdeletion and the resulting sperm defect, microdeletions in AZFa lead to Sertoli cell-only syndrome (SCO), mutations in AZFb provoke an interruption in meiosis I, and mutations in AZFc result in hypospermatogenesis, progressing to severe azoospermia or oligozoospermia (Ferrás et al., 2004b; Foresta et al., 2005). Microdeletions in the AZF region are frequently found in patients with azoospermia. The incidence of these microdeletions has been found to vary from 3 to 55% in Yq of patients with infertility problems (Foresta et al., 1998; Vogt, 2004). A relatively high frequency of "de novo" deletions could be due to spontaneous susceptibility to loss of genetic material in the Y chromosome. Although a high percentage of infertile men with microdeletions in the Y chromosome are not able to produce children by natural mechanisms of reproduction, there can be transmission of the father’s infertility problems to his sons, when they are produced by assisted reproduction. This predisposition for infertility can include gradual alterations in spermatozoid production, so that a young man with oligozoospermia later becomes azoospermic (Kihaile et al., 2005). Assisted reproduction techniques can help certain patients get pregnant, but infertility characteristics are still transmitted to their children. We examined microdeletions in the Y chromosomes of patients with azoospermia and severe oligozoospermia in an assisted reproduction clinic in the Goiânia city, west-central of Brazil. MATERIAL AND METHODS Patients The project was approved by the Ethics in Research Committee of the Catholic University of Goiás (No. 150/2004). An informed consent form was signed by all of the participants. Samples of blood and semen were collected from 300 men at the Laboratory of Human Reproduction (HC-UFG) in 2004, 2005 and 2006; only those with normal karyotypes were selected. The patients were classified according to alterations detected in three consecutive spermograms, based on the WHO technique (1999), into groups with non-obstructive azoospermia and those with severe oligozoospermia (≤5 x 106 sperm/mL). DNA isolation and molecular analysis Genomic DNA was isolated from peripheral blood lymphocytes or semen following the instructions of the GFXTM Genomic Blood DNA purification kit (Amershan Pharmacia Biotech Inc.®, USA). Microdeletion analysis was made of the regions AZFa, AZFb and AZFc sequence-tagged sites (STS; Table 1). The amplification system that we used, recommended by the European Academy of Andrology (EAA), allows us to detect 90% of the microdeletions in the AZF region (Simoni et al., 1999, 2004). For positive control samples, we used genomic DNA from fertile men (normal spermograms) with naturally conceived children; negative control DNA was obtained from women. The SRY gene (sex-determining region of the Y) was examined in the control group to confirm the sex and to look for ZFX/ZFY genes (zinc finger transcription factor). The PCR product was run by electrophoresis on a 1.5% agarose gel impregnated with ethidium bromide at 5 µg/mL and visualized under UV light. The STS region was considered absent after three repetitions with negative results.
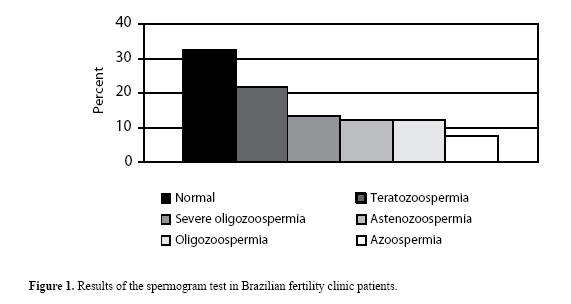
RESULTS We selected 63 of the original 300 patients who had azoospermia or non-obstructive or severe oligospermia (21%). We found 65 infertile males based on spermogram alterations (21.6%) with teratozoospermia and 37 (12.3%) with asthenozoospermia. Alterations in the number of cells produced were found in 13.3% due to severe oligozoospermia (<5 x 106 sperm/mL), 12.3% due to oligozoospermia and 7.6% due to non-obstructive azoospermia (Figure 1).
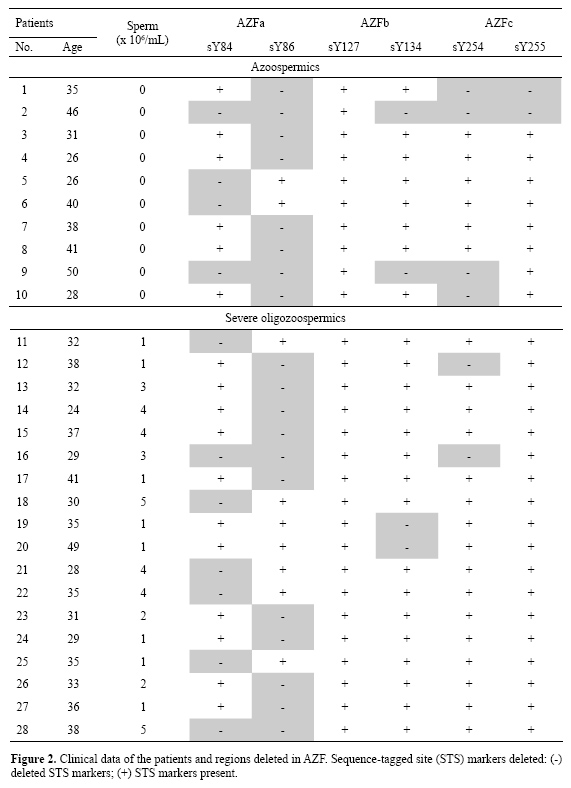
Among the 63 patients with azoospermia or severe oligozoospermia, 28 had microdeletions, 10 were azoospermic patients and 18 had severe oligozoospermia. The ages of the azoospermic patients varied from 23 to 50 years, with a mean of 36 years. Patients with severe oligozoospermia ranged from 21 to 62 years, with a mean of 35 years. Among the 23 azoospermic patients, 10 had deletions in the AZFa region, 2 had microdeletions in the AZFb region and 4 in the AZFc region. Among the 40 severe oligozoospermic patients, 16 had deletions only in the AZFa region, 2 had microdeletions in the AZFb region and 2 in the AZFc region (Figure 2).
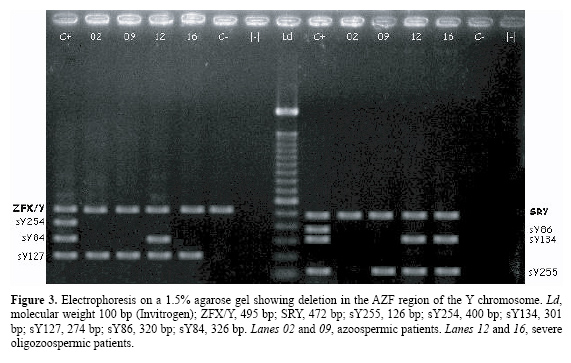
Amplification of the genes SRY and ZFX/ZFY was detected in all the patients and in the positive controls, while only the ZFX/ZFY amplified in the negative control (Figure 3).
DISCUSSION Studies on microdeletions of the Y chromosome have been carried out with a variety experimental designs, and variation in the prevalence of the microdeletions in the Y chromosome from 1 to 55.5% has been reported. A number of factors have been proposed to explain the wide variation in Y deletion frequencies, including population, ethnic variation, environmental influence, patient selection criteria, STS, and classification values used to define severe oligospermia of 5 x, 2 x or 1 x 106 sperm/mL. Blood or semen DNA was used. There is no consensus about the marker that should be used for Y chromosome microdeletion analysis (Foresta et al., 1998; Lê Bourhis et al., 2000; Krausz et al., 2001; Loginova et al., 2003; SãoPedro et al., 2003; Carrara et al., 2004; Dada et al., 2004; Simoni et al., 1999, 2004; Vogt, 2004; Hellani et al., 2006; Pina-Neto et al., 2006). SãoPedro et al. (2003) utilized the same values that we used for severe oligozoospermia; however, Vogt et al. (1996) considered the values for severe oligozoospermia below 2 x 106 sperm/mL. Carrara et al. (2004) and Hellani et al. (2006) established values for severe oligozoospermia of <1 x 106 sperm/mL. Dada et al. (2004) only indicated oligozoospermia without specifying the number of spermatozoa. Some authors, including Krausz et al. (2001) and Loginova et al. (2003) considered a spermatozoa number below 1 x 106 sperm/mL to be cryptozoospermia. Deletions in the AZF region are commonly found in patients with azoospermia, which we also found in our study, but a genotype-phenotype correlation has not been objectively demonstrated. Deletions in the AZFb region have been found to be associated with azoospermia, oligozoospermia, and normozoospermia; deletions in the AZFc region have been found to be associated with azoospermia and severe to mild oligozoospermia (Thangaraj et al., 2003). In our country, studies made by SãoPedro et al. (2003) detected 6.7% microdeletions in patients in São Paulo city, based on 14 STS, with 6.8% azoospermics and 6.4% severe oligozoospermics. But they did not analyze Y134; we observed 14.2% microdeletions with this STS. However, Carrara et al. (2004) also reported a prevalence of 5.3% microdeletions within the AZFc region of infertile Brazilian men using 28 STS and none of these STS were considered by EAA, and they found no deletions in SCO-affected patients; but Pina-Neto et al. (2006) found 7.5% microdeletions in all three AZF regions using the same 28 STS used by Carrara et al. (2004) in the same hospital. All these studies were made in the southeast region of Brazil. The difference in the deletion prevalence that we found could be due to the characteristics of the region where the study took place, this study being the first to examine these factors. However, in both regions there is an Italian contribution; Foresta et al. (1998) reported a microdeletion in the AZFa region in over 55% of Italian patients with SCO. We did not make a histological diagnosis of our patients. Molecular studies are underway for extension analysis of the microdeletions in the AZFa region. Our study region was predominantly rural; this could explain testicle accidents due to falling from horses along with problems due to pesticide handling. We also found that 52% of the azoospermic patients who had presented microdeletions in the AZFa region reported testicle accidents with wounds and/or mumps. Dejucq and Jégou (2001) observed testicle atrophy in 40 to 70% of patients with orchitis; this influences the production of spermatozoids, resulting in azoospermia. Unilateral involvement is more common than bilateral, which occurs in 15 to 30% of couples; even though it is evident that, in contrast with what was expected, external causes are responsible for unexplained microdeletions in the Y chromosome. Studies suggest that deletions in AZFa can be events of recombination between specific repetitive regions defined as hot spots (Kamp et al., 2001). These comparative observations led us to believe the hypothesis that some molecular mechanism, operating on defined hot spots in Yq, could be responsible for a similar recurrence of AZFa deletions. The loci STS sY84 and sY86 used in the EAA minimal set for analysis of AZFa deletions are always deleted in patients with complete AZFa deletions (Simoni et al., 1999, 2004); however, studies relate absence of microdeletions of these markers in the azoospermic population and suggest that these deletions are associated in some populations but not in others, as with sY746 in the same region (Pena et al., 2000; Krausz et al., 2003; Thangaraj et al., 2003; Fernandes et al., 2004). Deletions would have been noted if markers were used for a specific population. It is not clear how to diagnosis the cause of infertility in patients. Studies of microdeletions in Yq will help in the development of better methods of diagnosis and will be useful for increasing our understanding of spermatogenesis. Molecular examinations of genetic causes should not exclude the preliminary questioning of the couple. However, such studies are necessary to determine if microdeletions in chromosome Y are related to external factors that could be causes of "de novo" mutations in patients. There is an urgent necessity for implementing molecular methods in medical clinics. Diagnosis of genetic alterations and knowledge on vertical transmission of these abnormalities are essential for studies of infertile men who participate in assisted human reproduction. ACKNOWLEDGMENTS The authors acknowledge financial support from Universidade Católica de Goiás, Goiânia, GO, Brazil (UCG/PROPE) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). References Carrara RCV, Yamasaki R, Mazucatto LF, Veludo MAL, et al. (2004). Somatic and germ cell cytogenetic studies and AZF microdeletion screening in infertile men. Genet. Mol. Biol. 27: 477-482. Dada R, Gupta NP and Kucheria K (2003). Molecular screening for Yq microdeletion in men with idiopathic oligozoospermia and azoospermia. J. Biosci. 28: 163-168. Dada R, Gupta NP and Kucheria K (2004). Yq microdeletions - azoospermia factor candidate genes and spermatogenic arrest. J. Biomol. Tech. 15: 176-183. Dejucq N and Jégou B (2001). Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiol. Mol. Biol. Rev. 65: 208-231.Fernandes S, Paracchini S, Meyer LH, Floridia G, et al. (2004). A large AZFc deletion removes DAZ3/DAZ4 and nearby genes from men in Y haplogroup N. Am. J. Hum. Genet. 74: 180-187. Ferrás C, Costa P, Fernandes S, Carvalho F, et al. (2004a). Importância do estudo das microdeleções do cromossoma Y na infertilidade masculina. Acta Urol. 4: 17-26. Ferrás C, Fernandes S, Marques CJ, Carvalho F, et al. (2004b). AZF and DAZ gene copy-specific deletion analysis in maturation arrest and Sertoli cell-only syndrome. Mol. Hum. Reprod. 10: 755-761. Foresta C, Ferlin A, Garolla A, Moro E, et al. (1998). High frequency of well-defined Y-chromosome deletions in idiopathic Sertoli cell-only syndrome. Hum. Reprod. 13: 302-307. Foresta C, Moro E and Ferlin A (2001). Y chromosome microdeletions and alterations of spermatogenesis. Endocr. Rev. 22: 226-239. Foresta C, Garolla A, Bartoloni L, Bettella A, et al. (2005). Genetic abnormalities among severely oligospermic men who are candidates for intracytoplasmic sperm injection. J. Clin. Endocrinol. Metab. 90: 152-156. Hellani A, Al-Hassan S, Iqbal M and Coskun S (2006). Y chromosome microdeletions in infertile men with idiopathic oligo- or azoospermia. J. Exp. Clin. Assist. Reprod. 3: 1. Kamp C, Huellen K, Fernandes S, Sousa M, et al. (2001). High deletion frequency of the complete AZFa sequence in men with Sertoli-cell-only syndrome. Mol. Hum. Reprod. 7: 987-994. Kihaile PE, Yasui A and Shuto Y (2005). Prospective assessment of Y-chromosome microdeletions and reproductive outcomes among infertile couples of Japanese and African origin. J. Exp. Clin. Assist. Reprod. 2: 9. Krausz C, Rajpert-De ME, Frydelund-Larsen L, Quintana-Murci L, et al. (2001). Double-blind Y chromosome microdeletion analysis in men with known sperm parameters and reproductive hormone profiles: microdeletions are specific for spermatogenic failure. J. Clin. Endocrinol. Metab. 86: 2638-2642. Krausz C, Forti G and McElreavey K (2003). The Y chromosome and male fertility and infertility. Int. J. Androl. 26: 70-75. Le Bourhis C, Siffroi JP, McElreavey K and Dadoune JP (2000). Y chromosome microdeletions and germinal mosaicism in infertile males. Mol. Hum. Reprod. 6: 688-693. Loginova IA, Nagornaia II, Shlykova SA, Petrova LI, et al. (2003). Molecular genetic analysis of Y-chromosome micro deletions in men with severe spermatogenic defects. Mol. Biol. 37: 74-80. Pena SDJ, Silva DRC, Silva JA, Prado VF, et al. (2000). Retrato molecular do Brasil. Rev. Ciência Hoje 27: 159. Pina-Neto JM, Carrara RC, Bisinella R, Mazzucatto LF, et al. (2006). Somatic cytogenetic and azoospermia factor gene microdeletion studies in infertile men. Braz. J. Med. Biol. Res. 39: 555-561. Rao L, Babu A, Kanakavalli M, Padmalatha V, et al. (2004). Chromosomal abnormalities and y chromosome microdeletions in infertile men with varicocele and idiopathic infertility of south Indian origin. J. Androl. 25: 147-153. SãoPedro SL, Fraietta R, Spaine D, Porto CS, et al. (2003). Prevalence of Y chromosome deletions in a Brazilian population of non-obstructive azoospermic and severely oligozoospermic men. Braz. J. Med. Biol. Res. 36: 787-793. Simoni M, Bakker E, Eurlings MC, Matthijs G, et al. (1999). Laboratory guidelines for molecular diagnosis of Y-chromosomal microdeletions. Int. J. Androl. 22: 292-299. Simoni M, Bakker E and Krausz C (2004). EAA/EMQN best practice guidelines for molecular diagnosis of Y-chromosomal microdeletions. State of the art 2004. Int. J. Androl. 27: 240-249. Thangaraj K, Gupta NJ, Pavani K, Reddy AG, et al. (2003). Y chromosome deletions in azoospermic men in India. J. Androl. 24: 588-597. Tiepolo L and Zuffardi O (1976). Localization of factors controlling spermatogenesis in the nonfluorescent portion of the human Y chromosome long arm. Hum. Genet. 34: 119-124. Vogt PH (2004). Molecular genetics of human male infertility: from genes to new therapeutic perspectives. Curr. Pharm. Des. 10: 471-500. Vogt PH, Edelmann A, Kirsch S, Henegariu O, et al. (1996). Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Hum. Mol. Genet. 5: 933-943. WHO (1999). World Health Organization Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th edn. Cambridge University Press, Cambridge. |
|