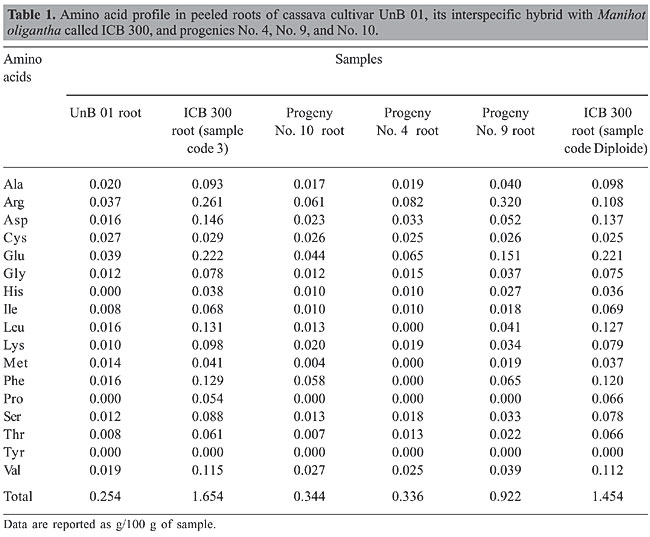
ABSTRACT. Cassava roots have a low-protein content (0.7-2%). Amino acids such as lysine and methionine are also low, and some research reports have indicated the absence of methionine. The amino acid profiles of a common cassava cultivar and an interspecific hybrid, namely ICB 300, were determined using the computerized amino acid analyzer Hitachi L-8500. The interspecific hybrid has 10 times more lysine and 3 times more methionine than the common cassava cultivar: lysine content was 0.010 g per 100 g in the common cassava cultivar while it reached 0.098 in the interspecific hybrid. Methionine in the common cassava cultivar was 0.014 g per 100 g whereas it reached 0.041 g per 100 g in the interspecific hybrid. Total amino acid content in the common cassava cultivar was 0.254 g per 100 g viz. a viz. 1.664 g per 100 g in the interspecific hybrid. The genetic variability of the profile and quantity of amino acids indicate the feasibility of selecting interspecific hybrids that are rich in both crude protein and amino acids. This is the first report of high true protein in cassava root. Key words: Amino acids, Cassava, Lysine, Methionine, Protein content, Interspecific hybrids INTRODUCTION Cassava roots provide more than 60% of the daily energy intake for the population of Northeastern Brazil and many countries in Africa. Cassava roots, however, are a poor source of protein, despite that the quality of this protein is fairly good, as is the proportion of amino acids as well. Methionine and lysine are, however, limiting acids in the root. If varieties could be developed with a higher quantity of these amino acids, it would enhance the value of cassava as a food and/or feed. Only about 60% of the total nitrogen are derived from amino acids, and about 1% of it is in the form of nitrates and hydrocyanic acid. The remaining 38-40% of the total nitrogen remain unidentified (Diasolua et al., 2002, 2003). Cassava protein is comparable to rice protein in digestibility. The biological value (Block and Michell equivalent) of the total protein is 48%. The crude protein content of roots appears to be relatively stable and constant with maturity of the plant. According to Close et al. (1953), the protein of processed cassava includes the highest percentage of glutamic acid and the lowest of methionine (1%). Sreeramanamubry (1945) reported the total absence of methionine, whereas Osuntokun et al. (1968) reported that both cystine and cysteine are involved in cyanide detoxification. Cyanide is produced when the glycoside linamarine is hydrolyzed by linamarinase. MATERIAL AND METHODS Samples Root powder was taken from a common cassava cultivar namely UnB 01, the interspecific hybrid between cassava x Manihot oligantha root, designated ICB 300 Diploide, the first progeny of the interspecific hybrid ICB 300 root, designated Progeny 4, a second progeny of the hybrid ICB 300 root designated here Progeny 9, a sample of the interspecific hybrid ICB 300 designated here sample 3, and the progeny of ICB 300, designated here Progeny 10. Sample extraction Samples (500 mg) of cassava root powder were extracted with 1 mL 10 mM HCl for 4 h, at 25°C, under agitation at 1,200 rpm in Thermomixer (Eppendorf, Hamburg, Germany). The suspensions were then centrifuged for 4 min at 6,000 rpm in a bench centrifuge. The supernatant (800 µL), called acid extract, and the remaining powder were dried down in a SpeedVac vaccum centrifuge (Savant, NY, USA). The dried powder was extracted in the same way with 1 mL 10 M NH4OH producing an alkaline extract. The dried acid extract was resuspended with 750 µL 10 mM HCl, washed with an extra 750 µL of the same dilute acid, and added to 750 µL of the alkaline extract. The total extract was exhaustively dialyzed against MilliQ water and vacuum-dried in a SpeedVac centrifuge. Amino acid analysis Aliquots of 150 µg of each extract were dissolved in 75 µL 100 mM HCl. Acid hydrolysis of the samples was performed in 6 M HCl under vacuum for 24 h at 109°C. After acid hydrolysis, the hydrolyzed samples were solubilized in 75 µL 100 mM HCl, and 50 µL was injected into an amino acid analyzer (Hitachi L8500, Tokyo, Japan). The analyses for determination of amino acid compositions were performed in triplicate. The total protein contents of the samples were calculated by summing up the amounts of the amino acids. RESULTS AND DISCUSSION Amino acid compositions from Manihot proteins were determined by analyzing sample extracts which were dialyzed with water to remove free amino acids, salts, monosaccharides, and other small molecules. Tryptophan could not be analyzed since it is degraded upon acid hydrolysis. By summing the amounts of the analyzed amino acids, it was possible to determine the protein content for each sample. Among the six samples analyzed in this study (Table 1, Figure 1A-D), the sample of the interspecific hybrid ICB 300 denominated sample 3 showed the highest amount of protein (1654 g/100 g sample powder), followed by the ICB 300 sample denominated Diploide (1454 g/100 g) and ICB 300 Progeny No. 9 (0.922 g/100 g). The samples of ICB 300 Progeny No. 10, ICB 300 Progeny No. 4 and UnB 01 Raiz were poorer in protein content (0.350 g/100 g). The levels of essential amino acids were also higher in sample 3 of the hybrid ICB 300 (His, Leu, Lys, Met, Phe, and Val) and sample Diploid of the same hybrid (Ile and Thr), with low or undetectable amounts in the other cultivars. Thus, these two samples of this interspecific hybrid ICB 300 show that the interspecific hybrid would be more interesting for human consumption based on such nutritional characteristics.
The hybrid ICB 300 was produced by crossing cassava with the wild species M. oligantha, which contains 4% crude protein (Nassar and Dorea, 1982). Its Progeny No. 9 showed an equal amount of protein, i.e., double that of common cassava indicating high hereditability of this character and the possibility of selecting for high-protein cassava. There is agreement in the literature on the very low percentage of the amino acids methionine and lysine. Essential amino acids were reported by Sreeramanamubry (1945) to be found in cassava. These amino acids are arginine, histidine, isoleucine, leucine, phenylalanine, threonine, tryptophan, and valine. Methionine and tryptophan were deficient. The results of this research regarding amino acids in cassava roots agree with those from the available literature. For example, Bailey (1961) indicated a deficiency in sulfur-containing amino acids (methionine, cystine and cysteine). Osuntokun et al. (1968) pointed out that both cysteine and cystine are involved in cyanide detoxification (cyanide is produced when the cyanogenic glucoside linamarine present in cassava is hybrolyzed by linamarinase or by acid). Cyanide is mainly detoxicated by conversion to thiocyanate, in the process of which it reacts with cysteine and cystine. Excessive detoxification may be responsible for the low concentration of sulfur-containing amino acids. Efforts have been made in the past to increase the protein content of cassava roots by interspecific hybridization with a wild species, namely M. saxicola and M. melanobasis. Over a period of 10 years beginning in 1932 and ending with the Japanese occupation of Java in 1942, Bolhuis (1953) carried out a program of cassava breeding for increased protein content in roots. Crosses with M. saxicola yielded a few seedlings with as much as 2% protein in the root fresh. In the clones, he propagated from these seedlings protein content fell back to typical levels. Jennings (1959) reported that the roots of the F1 progeny of M. esculenta x M. melanobasis possessed approximately twice as much protein as their cassava parent. Such progenies were, however, lost and not grown anywhere, probably because of poor root yield. Some researchers such as Barros and Bressani (1967) and other researchers from the Centro Internacional de Agricultura Tropical (CIAT) in the 1970’s reported cassava cultivars with high-protein content as much as 7%, but it is doubtful if the nitrogen matter referred to in these cultivars are protein or no more than breakdown products of cyanogenic glucosides. Therefore, according to Narthy (1968) it is not unlikely that these reported cultivars of high-nitrogenous content turn out to be nothing but bitter cultivars with high-glucoside content. Humidity when drying the material is another factor that interferes with protein determination as total nitrogen. Excessive drying of the root powder may increase drastically the percentage of nitrogen material by 3-fold. Hence, it is important to determine protein content as amino acids jointly with its assessment as nitrogen matter. Description of the hybrid ICB 300 Shrub is 2-3 m high. Roots are conical, 40-60 cm, having a neck 3-4 cm, and they are abundant, 5-10 kg per plant. Root surface color is dark brown, root flesh white, stem brown, story 1-1.2 m high. Scars on stem are moderately raised. Stems are 3-branched. Leaves have 3 lobes, occasionally 1 or 4 lobes. Leaf lobe is obovate, with simple margins and green reddish petioles. Young foliage at apices is green. Inflorescence is a panicle 10-20 cm glabrous, with bracts and bracteoles inconspicuous and caducous. My original is right. The Botanical description has its specific style, tends to abbreviation as possible as. Flowers are monoecious, pistillate based 2-3, open 3 weeks earlier than staminate ones. Perianth has 5 separate tepals, yellow pistillate ovary has ovary sublended by a non-lobed yellow disk, 3 carpellate, glabrous. Fruit is winged and smooth surfaced. Seeds are caranculate elongate and brown. REFERENCES Bailey KV (1961). Rural nutrition studies in Indonesia. II. Clinical studies of hunger oedema in the cassava areas of Java. Trop. Geogr. Med. 13: 234-254. Barros EA and Bressani R (1967). Composicion química de la raiz de la hoja de algunas variedades de yuca Manihot (Chemical composition of the roots and leaves of some varieties of Manihot). Turrialba 17: 314-320. Bolhuis GG (1953). A survey of some attempts to breed cassava varieties with high content of protein in the roots. Euphytica 2: 107-112. CIAT (1971). Annual report. CIAT, Cali Close J, Adrianens EL, Moore S and Bigwood EJ (1953). Composicion en acides amines d’hydrolyts de farine de manioc variete. Am. Bull. Soc. Chem. Biol. Brassels 35: 985. Diasolua Ngudi D, Kuo Yu-Haey and Lambein F (2002). Food safety and amino acid balance in processed cassava “Cossettes”. J. Agric. Food Chem. 50: 3042-3049. Diasolua Ngudi D, Kuo Yu-Haey and Lambein F (2003). Amino acid profiles and protein quality of cooked cassava leaves or ‘saka-saka’. J. Sci. Food Agric. 83: 529-534. Jennings DL (1959). Manihot melanobasis Muell. Arg-a useful parent for cassava breeding. Euphytica 8: 157-162. Narthy F (1968). Studies on cassava cyanogenesis: the biosynthesis of linamarin and lotaustralin. Phytochemistry 7: 1307-1312. Nassar NMA and Dorea G (1982). Protein contents of cassava cultivars and its hybrid with Manihot species. Turrialba 32: 429-432. Osuntokun BO, Durowoju JE, McFarlane H and Wilson J (1968). Plasma amino-acids in the Nigerian nutritional ataxic neuropathy. Br. Med. J. 3: 647-649. Sreeramanamubry VV (1945). Investigations on the nutritive value of tapioca (Manihot utilissima). Indian J. Med. Res. 33: 229-238. |
|