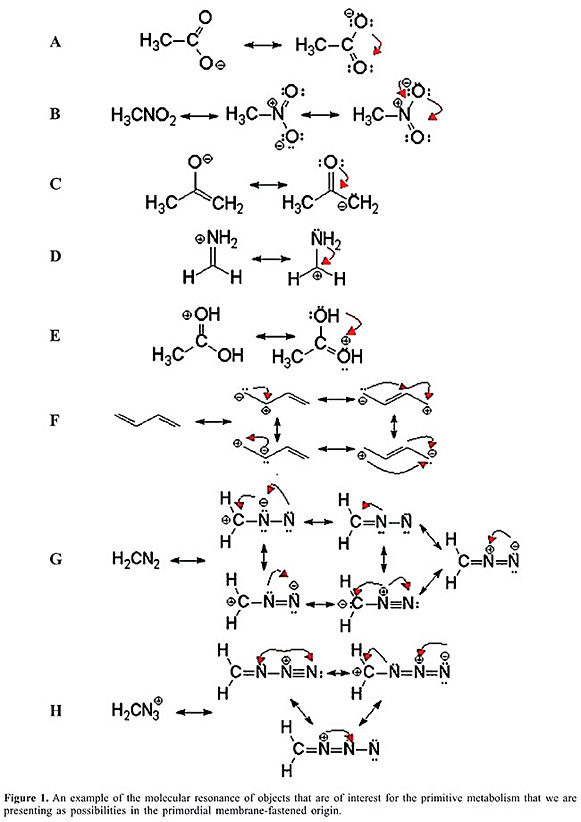
ABSTRACT. The present study is just an overview of the opening of the geochemical stage for the appearance of life. But that opening would not have been sufficient for the intellectual discovery of the origin of life! The excellent works and many commendable efforts that advance this explanation have not shown the fundamental elements that participate in the theoretical frame of biological evolution. The latter imply the existence of evolutionary transitions and the production of new levels of organization. In this brief analysis we do not intend to introduce the audience to the philosophy of biology. But we do expect to provide a modest overview, in which the geochemical chemolithoautotrophic opening of the stage should be seen, at most, as the initial metabolism that enabled organic compounds to follow the road where a chemical fluid machinery was thus able to undertake the more “sublime” course of organic biological evolution. We think that Tibor Gánti’s chemoton is the most significant contribution to theoretical biology, and the only course now available to comprehend the unit of evolution problem without the structuralist and functionalist conflict prevalent in theoretical biology. In our opinion Gánti’s chemoton theory travels to the “locus” where evolutionary theory dares to extend itself to entities at many levels of structural organization, beyond the gene or the group above. Therefore, in this and subsequent papers on the prebiotic conditions for the eventual appearance of the genetic code, we explore the formation and the presence of metal sulfide minerals, from the assembly of metal sulfide clusters through the precipitation of nanocrystals and the further reactions resulting in bulk metal sulfide phases. We endeavor to characterize pristine reactions and the modern surfaces, utilizing traditional surface science techniques and computational methods. Moreover, mechanistic details of the overall oxidation of metal sulfide minerals are set forth. We hope that this paper will lead our audience to accept that in a chemically oscillating system the chemoton is a model fluid state automaton capable of growth and self-reproduction. This is not simply a matter of transmitting a pattern, as in inorganic crystals; such self-reproduction must be more complex than crystal growth. Indeed that is what Gánti’s theoretical and abstract model offers to us all: we finally have a philosophy of evolutionary units in theoretical biology. Key words: Prebiotic, Chemical fluid machinery, Biological evolution THE GEOCHEMICAL ORIGIN Below the Hadean Ocean crust, geological evidence estimated at some 4.2 gigas ago presents the conditions necessary for a pristine flow of energy from a redox energy source where 2-D metabolism could have emerged at a very specific site in the hot (ca. 100°C) extremely reduced, alkaline, iron-bisulfide found in submarine seepage waters (Wächtershäuser, 1988b, 1990, 1994). But this is not the origin of life. In fact, evidence is growing that not only hydrothermal fluids along mid-ocean ridges may be common (generally located on young crust driven hydrothermal flow), but also, in accordance with recent sea-floor studies, lower temperature venting associated with older tectonized portions of the oceanic crust (German et al., 1996; German and Parson, 1998; Barriga et al., 1998; Gracia et al., 2000). For us, the most recent observation of this type of low temperature venting associated with chimney development and serpentinization processes has been the discovery of the so-called lost city field, in short (Kelley et al., 2001), which opens new scenarios in organic-molecular formations. Serpentinization processes of the ridge system have focused attention on early Earth hydrothermal systems that generate significant CH4, H2, and as we know now, significant amounts of organic compounds during mineral-fluid reactions (Janecky and Seyfried Jr, 1986; Berndt et al., 1996; Allen et al., 1998; Shock and Schulte, 1998). This newly discovered class of sea-floor hydrothermal systems may provide insights into and confirmation of the fact that hydrothermal processes of the early Earth correlate well with the life forms that they supported (Schopf, 1983). This is the general scenario for Huber and Wächtershäuser’s (1997) modeling of the reactions of the reductive acetyl-coenzyme A pathway at hydrothermal temperatures and oceanic crust conditions in which coprecipitated NiS and FeS converted CO and CH3 SH into activated thioester CH3 - CO - SCH3 which hydrolyzed to acetic acid. Mid-ocean ridges located in the young crust where cooling of hot basaltic rock provokes hydrothermal flow is the right scenario for Huber and Wächtershäuser (1997)’ precipitation of iron and sulfide-rich mounds to occur during mixing of 200-350°C hydrothermal fluids with cold and more oxygenated sea water. EXTANT LIFE WITHIN ACTIVE FUMING DEEP-SEA OCEANIC CHIMNEYS The ecology of deep-sea hydrothermal vents has been eloquently reported (Van Dover, 2000). However, the recent study of the amazing lost city field does not report macrofauna, with the exception of a few crabs, sea urchins, sponges, and corals - only dense microbial species are found, sometimes in communities of abundant biomass. According to the preliminary report (Kelley et al., 2001) and analysis, early extractions of DNA indicate that both archaeal and eubacterial lineages are present at lost city. Three hundred and sixty-Myr-old hydrothermally formed iron sulfide chimneys were first discovered at Silvermines, Ireland (Larter et al., 1981; Boyce et al., 1983). Others, found in sulfide deposits within the ophiolite area of Cyprus (Oudin and Constantinou, 1984) and Oman (Haymon et al., 1984), show fossils that were extant 100 Myrs ago around ridge crest hot springs. Banks (1985) found in Tynagh, Ireland 350-Myr-old fossils recovered from pyrite chimneys associated with sedimentary-exhalative mineralization. Figure 2, in Martin and Russell (2002), shows iron monosulfide precipitates (Fe S), which oxidized to pyrite (Fe S2), and which hydrothermally formed iron sulfide chimneys. Some of the chimneys contain sphalerite (ZnS) which, according to the brilliant hypothesis of Banks et al. (2002) were formed through the hydrothermal exhalation of metal-bearing fluid, originally derived from sea water filtered through the crust. Such was the beginning of the excitement of the possible evolutionary importance of thermal spring sites as chemically reactive “hatcheries” for the origin of life (Corliss et al., 1981; Russell et al., 1988). THE THEORIES ABOUT THE BIOGEOCHEMICAL ORIGIN OF PROTOCELLS The prebiotic broth theory as a modular model of Darwin, Oparin and Haldane (Darwin, 1887; Oparin, 1924; Haldane, 1929) can be considered in modern terms as a heterotrophic origin of life. Miller and colleagues are the modern proponents of that theory (Miller, 1953, 1955; Miller and Bada, 1988). The theory of a heterotrophic beginning assumes a slow accumulation of amino acids, bases, sugars, lipids, nucleic acids, and some groups of polypeptides, building more and more complex organic compounds in droplets (coacervates) concentrated, as it were, in a rich broth in some primitive ocean when the physical conditions of the planet permitted. The heterotrophic theory considers that such compounds self organized and finally became reproducing entities. At the outset, this extraordinary theory was very exciting for many biologists. It is typically based on principles of solution chemistry; the reactions have to take place in a water phase. Such prebiotic chemistry is extant organic chemistry writ large. Many experts have criticized its logic and its thermodynamic incompatibility (Woese, 1979; Cairns-Smith, 1982), and also its improbable occurrence, on many grounds (Shapiro, 1984, 1986, 1988). Since this theory does not appear to offer a bridge between biology, chemistry and biochemistry, most present day theories have preferred other paths (Woese, 1979). We, like many others nowadays, bet on sufficient concentrations of simple reducing inorganic molecules in iron-sulfide precipitates of crystals in a seepage site with physical compartments isolated from the immediate environment in self-contained redox reactions under a hydrothermal submarine Hadean ocean floor. Such could have been the beginning of metabolism in status nascendi imprisoned in iron crystals for a long time, before other self-organized chemical and biochemical reactions placed the prisoner in a newer, less rigid cell wall. At the beginning, at the very first stages of evolution, we propose that there was no reproduction, no natural selection, no heredity, and no variability that could serve as units of selection and of evolution. In such beginnings the conditions for molecules to react under Gibbs conditions of thermodynamics spurred the reactions to proceed undeterred, far from equilibrium. Therefore, there was no origin of life at that time, because the ingredients were absent or incomplete. However, the chemistry for later life was represented in a group of readily made compounds that could respond to each other without catalysis. Thus, no enzymes, only mineral crystals precipitated as iron sulfide, and others, such as nickel sulfide, which in accordance with the laws of natural chemistry carry on redox reactions catalyzed by iron sulfur centers and by the presence of ferredoxins of simple organic compounds. These were mineral chemistry conditions, distinct from water chemistry, where newly made compounds could be washed away. The organic compounds were then fixed on a pyrite surface, enjoying free electronic energy in a reduced and alkaline condition and relatively high temperature and pressure, under the crust of the Hadean Ocean and in mounds of hydrothermal vents. Instead of natural selection, reactions depended on electronic sorting in the reticular “tissue” of the mineral with Fe S/H2 replacing coenzyme A in a reverse citric acid cycle. Indeed, in all extant organisms, transition metal sulfide clusters play an indispensable role in biological energy conversion systems (Spiro, 1997). The predominance of mineral sulfides in hydrothermal and volcanic vents (Larter et al., 1981; Boyce et al., 1983; Haymon et al., 1984; Oudin and Constantinou, 1984; Banks, 1985), preferably in pyrite chimneys associated with sedimentary exhalative mineralization, has led some to speculate that life may have emerged from such environments (Wächtershäuser, 1988a,b, 1992). Ever since then, successful experiments have been designed to model aspects of the primitive hydrothermal vent chemistry. They have revealed the intrinsic potential of alkyl thiols (Heinen and Lauwers, 1996) from reactions involving iron sulfides in the presence of CO. We can no longer ignore the most important question. What is life? (Schrödinger, 1944). In Gánti’s unit model of living systems there are three functionally dependent autocatalytic subsystems: the metabolic chemical cycle, the template polymerization cycle and the membrane enclosing all of them. The precise functioning of this automaton lies in the exact stoichiometric coupling of the three subsystems. We discuss them to some extent at the end of this paper. WE NEED TO HAVE ENERGY CLEARING FOR THE MOLECULAR ORDER AND CHEMICAL ENERGY The prebiotic environment had abundant energy sources, but only a few can be candidates for the origin of life. Some have argued (Morowitz, 1992) that metabolism could have developed from very specific reactions that were spontaneous in early prebiotic conditions but that are enwrapped today in a series of constrained enzyme-catalyzed reactions. In brilliant experiments, Morowitz’s group (1995) noted that nitrogen can be brought into today’s metabolism through reductive amination, enzymatically catalyzed by glutamate dehydrogenase aided by NADPH as the electronic carrier, and they demonstrated that the synthesis of glutamate can be done very simply, without intervening enzymes, from a-glutaric acid in the presence of ammonium salt, with formate as the reducing agent. Indeed, Miller and Orgel (1974) had already concluded that the major modern metabolic pathways presumably evolved from very simple reactions in prebiotic milieu. We explore three attractive energy sources for early microorganisms. Pyrophosphate bond energy At least one pyrophosphate has been found in the structure of canaphites (Ca Na2 P2 O7 · 4 H2O; Rouse et al., 1988); it is the first case of mineral pyrophosphate; therefore, it could have been present in the early environment of the Earth. Another argument in favor of the presence of pyrophosphate as an early source of energy is its ubiquitous use in today’s organisms. Baltscheffsky (1971, 1977) has argued in favor of pyrophosphate-bond energy. Pyrophosphate-bond energy readily forms when the inorganic phosphate is dried and submitted to temperatures in the range that generally appear under hydrothermal conditions. Glyceraldehyde Several authors prefer other prebiotic sources of energy (Weber, 1987; Weber and Hsu, 1990). For the origin of glycolysis and for condensation reactions in prebiotic times, glyceraldehyde and glyceric acid esters are thought to be unique as key molecules in the chemical evolution leading to early metabolism. The fact is that formaldehyde in the formose reaction can synthesize glyceraldehyde. Once synthesized, glyceraldehyde can be incorporated in a variety of reactions that address early biochemical and bioenergetic processes. Several pathways promoted by glyceraldehyde or intervened by it can produce compounds, like glycerol, tetroses and alcohols, central to glycolysis. Weber (1989) has argued that such routes could possibly evolve into modern metabolism. Notwithstanding some criticisms about the connection to the primary reactions of prebiotic metabolism, Weber (1989) argues that glyceraldehyde itself can undergo polymerization into polyglyceric acid, and that in such a vest, it could act as catalytic and genetic molecules. Wächtershäuser’s pyrite As we have noted above, pyrite as a mineral crystal on whose surface electronic flows could fix CO, and therefore other organic molecules at hydrothermal vents in subterranean oceanic conditions, is the most likely possibility for a 2-D primordial metabolic-film-coating-pyrite-mineral surface. A variety of energetically and thermodynamically favorable reactions, including polymerization, and lipid chain synthesis from isoprene derivatives (Bloch, 1983, 1985), may originate from molecules that profit from pyrite metabolism. Pyrite, as we have argued before, does have a number of characteristics that make it an ideal candidate for the origin of prebiotic metabolic pathways, starting with CO fixation and simple formose reactions that end up in the well-known energetic features of glyceraldehyde. THE MEMBRANES OF PROTOCELLS THAT WERE NOT FREE At a certain moment, when the non-evolutionary units were still attached to the walls of the iron crystal that imprisoned protocellular biogeochemical reactions that are thermodynamically possible because they prevented their incipient autotrophic metabolism from hydrolyzing and slipping into the Hadean ocean, a new invention had to emerge as a new level of chemical compartment, although without intending to be a unit of evolution. We are referring to the bilayers of amphiphilic molecules as vesicles in the form of membranous films that become the dominant structure as concentrations increase further. The synthesis of lipids in what some have called 2-D fluids (Singer and Nicholson, 1972 in Gánti, 2003) and others have called self-assembled structures of amphiphiles (Deamer et al., 2002), corresponds to a new cell-wall fluid (Figure 1).
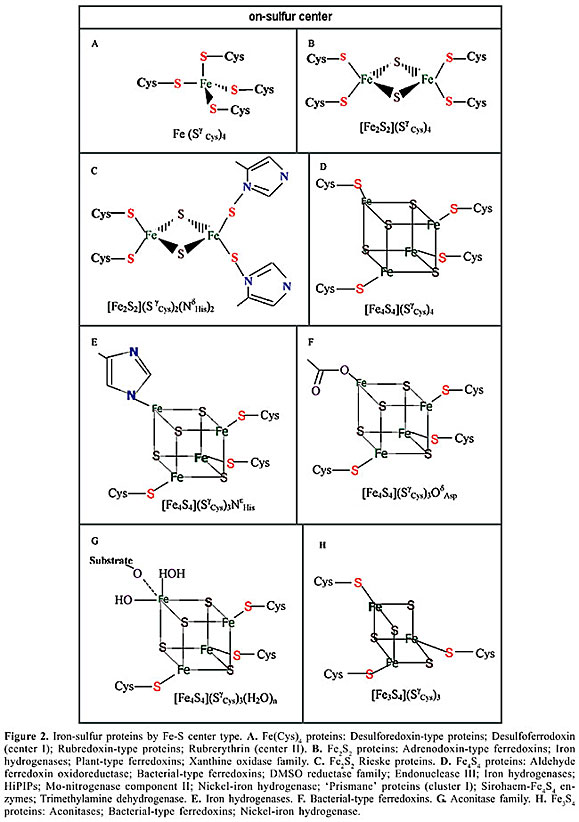
Both kinds of membranes could have done the job of isolating the cytosol from its FeS walls. But both required a new category of proteins, the ones that associate previous chemical evolution with redox chemistry. These would have been rich in centers of metal sulfides, so as to replace the functions originally associated with iron-sulfide redox chemistry. We show the modern molecular fossils of protein inventions with metal sulfide centers (Figure 2). The modules of electron transport proteins that are membrane-associated electron transport chains alla Baymann could constitute chemical replicators. But they are not the only chemical replicators, and they are not units of Dawkins’ replicators either.
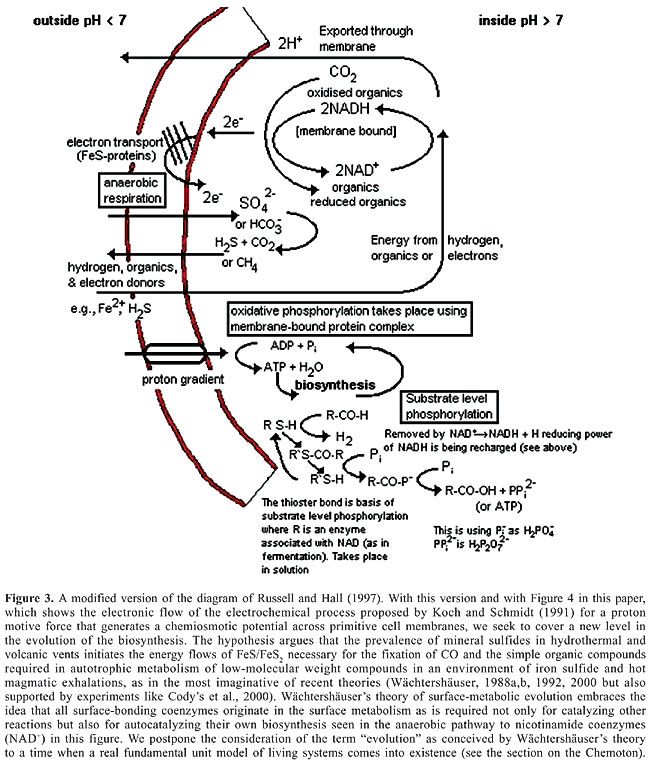
Therefore, the catalysis of (Fe >> Ni)S or other similar metal-sulfides (Russell et al., 2003) would separate from the metal wall while gradually incorporating to proteins. The imprint of this early feast is what we find in the molecular protein fossils that we show in Figure 2. What we are exploring here is the possibility that mineral imprints that preserve electronic transport jumped to a new level of biochemical evolution by converting themselves into protein modules, like ferredoxins and hydrogenase. In fact, modern protein modules of ferredoxins and hydrogenase participate in membranes frequently associated with electron transport chains (Baymann et al., 2003). What we propose as a tentative hypothesis is that the proteins that best responded to electron and proton transport through their particular resonance structure had the “choice” of fastening themselves to the best nearby mineral, simply because they constituted informative material (made up of abiotic amino acids) now constrained in their new function. The new chains were a protein module whose sequence would serve as a communication system able to transmit the best electron transport. Thus, glycolysis would have had a chance to create autotrophic respiration cemented to the membrane system (see Figure 3). This would be the first information system to transport the most expedient electron flow to preserve order and energy and to give permanence to the new phase in free-living microorganisms. Nucleic acids had not made their appearance yet. Repeatability in the biochemical functions was made through proteins, but this slow mechanism still awaited a faster one through a battery of enzymes and modern coenzymes, backed up by a real nucleic acid module.
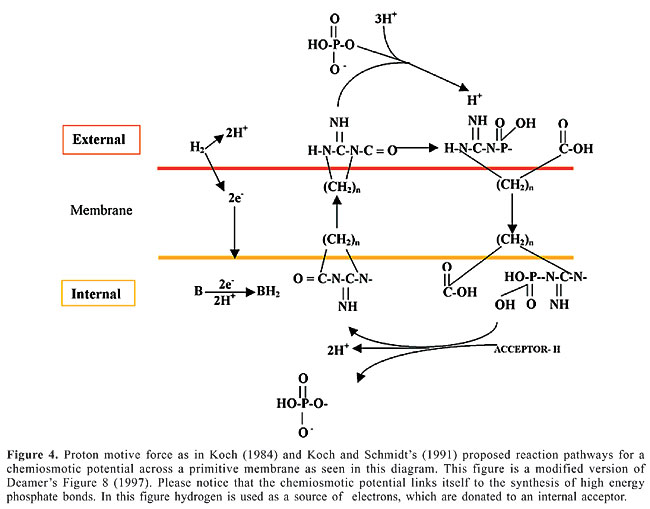
IONIC POTENTIALS ACROSS MEMBRANES For the basis of this section, we owe much to several investigators who have suggested that early protocells could have built ionic potentials from which energy in the form of a chemiosmotic force would emerge (Koch, 1984; Morowitz et al., 1988; King, 1990; Koch and Schmidt, 1991; Morowitz, 1981, 1992; Deamer, 1997). We are talking about a membrane electrochemistry that preceded translation, as King’s (1990) mind-boggling hypothesis rightly wants us to believe. Koch and Schmidt (1991) propose a hypothetical mechanism that would generate a chemiosmotic potential with a linkage to the synthesis of high-energy phosphate bonds (see Figure 4). Hydrogen is used as a source of electrons that flow inside to an internal acceptor. Their brilliant hypothesis contemplates a separate second reaction, in which external inorganic phosphate forms a bond with a molecule analogous to creatinine. The latter, as is well known, is linked to a hydrocarbon moiety (see the lower part of the figure for the link to the (CH2)n moiety), which then can pass through the membrane. The charges are neutralized on the phosphate, and the positively charged phosphorylated compound can then be electrochemically transported through the gradient to the cell interior. The cycle completes itself when the phosphate is transferred to an acceptor and then the neutral molecule slips back across to begin a new cycle. The ingenious mechanism ends up with an acceptor on the interior of the cell that serves as a source of high-energy phosphate to drive metabolic reactions. We have gotten to the point of asserting that when a sufficient and efficient battery of redox carriers has been assembled into the incipient membrane (as we explained before) to allow for the compartments to sustain ATP synthesis via chemiosmosis of the electrochemical constituents (as explained before by Koch and Schmidt, 1991 and Russell and Hall, 1997), we can expect to get cellular freedom from the mineral housing as long as we embrace the three autocatalytic subsystems of the chemoton: the metabolic chemical cycle, the template polymerization and the membrane subsystem enclosing them all. The only thing missing from Apel, Deamer and Mautner’s (2002) ingenious self-assembled model is that self-reproducing vesicles cannot be a minimal living system until they have an information subsystem.
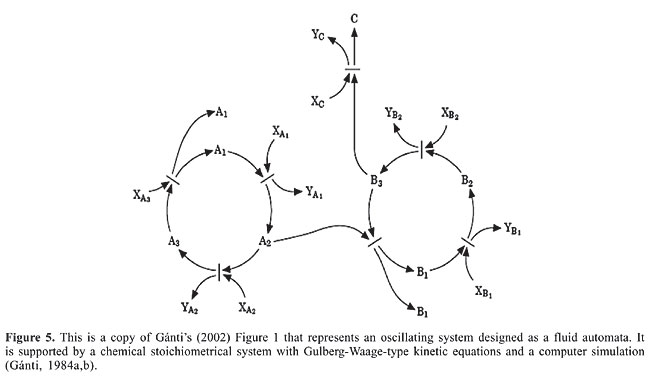
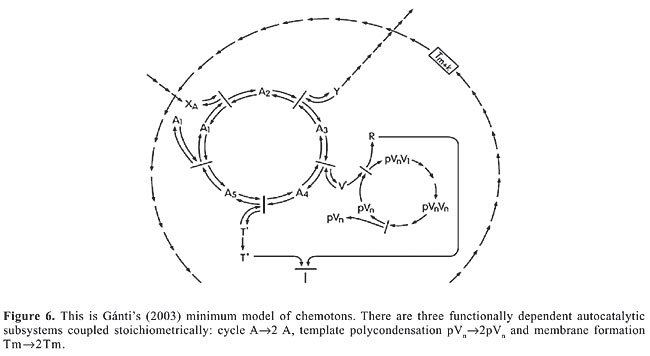
MEMBRANES FOR CELLULAR FREEDOM Martin and Russell’s model (2002) for the independent origin of membrane-connected compartmentalization of prokaryotes foresees that the two groups should differ substantially. In fact, although the two groups (archaebacteria and eubacteria) do possess isoprene side chains in their electron- and proton-transporting quinones (Schutz et al., 2000; Berry, 2002), these isoprenes are synthesized by different and unrelated biochemical pathways in the two groups. Moreover, the proton- and electron-transferring aromatic heads of the quinones also differ in these two groups of prokaryotes. THE CHEMOTON So far we have shown our inclination to favor a chemoautotrophic origin for the first protocellular system (Wächtershäuser’s theory, 1988a,b). Although geochemical processes necessary to the progressive chemical developments that led to the first physicochemical scenarios could have merits that other explanations lack, for a coherent definition of the origin of life on this planet we are not satisfied to merely get technical requirements out of the way. In the absence of direct observational elements, an explanation of the origin of life in the Archean (4.3-3.5 gigas) undoubtedly requires, not only a proper theoretical approach, but also a more satisfactory philosophical consideration (Gánti, 2003). Credible arguments for or against the temperature milieu theory (hyper or meso) are at best contentious. We appreciate that the thermal history of the Archean oceans (4.3 gigas) is an important element to establish the settings and conditions for chemical evolution and the origin of life, but there are other significant aspects of the general scenario, such as the fact that conditions for a continuous flow of energy in hot submarine seepage waters - high temperature (around 100°C) and pH, and extremely reduced alkalinity - could have been a threat to life unless the origin of life came in from other last universal common ancestor. Several geophysical arguments support the hypothesis of a primitive hot environment, but this is a contentious topic. Remembering the lower intensity of the sun around 4.2 gigas ago, Bada et al., 1994, argue that the primitive earth had frozen oceans occasionally thawed by the impacts of giant meteorites and warmed by hydrothermal activity. Nevertheless, we have to consider that last universal common ancestor could have come much later, maybe as the product of a long evolutionary history (Miller and Lazcano, 1995; Forterre et al., 1995). The low stability of RNA at high temperature forces a different template for information storage in a high-temperature environment (Edmonds et al., 1991; Forterre, 1995). There is even more of a challenge to a hyperthermophilic origin in the elaborated molecular devices that seem to be specific for thermoadaptation (Forterre, 1995). Having no geological evidence about the physical environment of 4.0 gigas ago, since there are no sedimentary rocks older than 3.8 gigas, it would be better for us to concentrate on the most important theoretical flaw about the real origin of life - to have left out of the solution Gánti’s chemoton model. Published by Eigen in 1971, two simple theoretical models, the hypercycle (Eigen and Schuster, 1979) and the chemoton (Gánti, 1971), attempt to cross not only the philosophical barrier but also the border that separates the living from the non-living systems. Not by chance, of course, both are fluid automaton models constructed from autocatalytic chemical systems, which by virtue of being fluid machines do not have to cope with geometrical impediments. The most ingenious of the two theoretical models is certainly the chemoton, not only because it can grow and divide as any cell would, but also because it is separated from the external environment and therefore possesses from its birth chemo-osmotic regulations due to the membrane that turns it into a supersystem with three autocatalytic cycles: 1) The first cycle is a self-reproducing metabolic cycle that transforms the external inflow of nutrients into the chemoton’s internal “cytoplasm” necessary for template replication and membrane growth. 2) The second subunit is a self-replicating cycle of a double-stranded template. The tip of the chain of the double-stranded “spiral” opens up as the result of fluctuations, but with high probability the monomers in both opposite strands reunite again without geometrical problems for the forms. 3) The third subunit is a model of a 2-D membrane that can be viewed as having a hydrophobic face and a hydrophilic face, which form a spherical enclosure of the system. In one of Gánti’s papers (Gánti, 2002), the circuit diagram of his Figure 1 is essentially the chemical equivalent of the Volterra and Lotka population dynamics of an oscillating system. In Figure 5 that follows, we present the coupling of two autocatalytic cycles, as in Gánti’s paper, in order to argue that the numerical integration of the differential kinetic equation system of that figure shows that the concentration of the intermediate compounds of the cycle do indeed oscillate. It is a periodically changing phenomenon! In the Lotka-Volterra system, the autocatalytic cycle A®2 A represents the population increase of hares, the B®2 B cycle represents that of the foxes that eat hares A2, and the B3®C may represent an analogy to the death of foxes! Gánti wrote down the Gulberg-Waage-type kinetic equations for each of the chemical reactions seen in Figure 6 that we reproduce below, and obtained a differential equation system which, when numerically integrated by computer enabled him to postulate a “ticking, machine”, i.e., the concentrations of the intermediate compounds of the cycle oscillate.
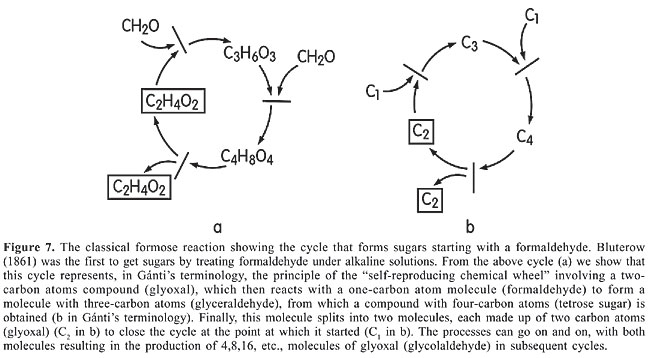
Leibniz (1705) in his “Principles of Life” and “Monadologie” (Leibniz, 1714) calls nature a divine constructor, which distinguishes man-made automata from nature’s own creations. The divine “mason” goes into the smallest units (as a fluid oscillatory machine, in Gánti’s terminology) while the artificial construct is an imperfect and limited contraption. Formaldehyde is the most common of organic compounds in the Universe, also present in the atmosphere of Hadean Earth. Atmospheric methane produced by solar UV, lightning, and radioactive and cosmic radiation produced formaldehyde constantly. Therefore, in view of the reactions shown in Figure 7, in which glyoxal and formaldehyde formation are sufficiently rapid, it would not be rash of us to say that the formose reaction must have been one of the most frequent self-reproducing chemical wheels on primordial Earth.
ACKNOWLEDGMENTS The author gratefully acknowledges Prof. Dr. Tibor Gánti for his corrections and kind opinions about this article. The author is particularly grateful to Tibor Gánti and his wife for stimulating conversations at Nagymaros, Hungary, last July 2005. Moreover, I thank Jimmy Weiskopf for his language corrections. REFERENCES Allen DA, Berndt ME, Seyfried WE Jr and Horita J (1998). Inorganic reduction of CO2 to HCOOH, CH4, and other reduced carbon compounds with application to subseafloor hydrothermal systems. Eos. Trans. Am. Geophys. Union 79: 58-59. Apel CL, Deamer DW and Mautner MN (2002). Self-assembled vesicles of monocarboxylic acids and alcohols: conditions for stability and for the encapsulation of biopolymers. Biochim. Biophys. Acta 1559: 1-9. Bada JL, Bigham C and Miller SL (1994). Impact melting of frozen oceans on the early earth: implications for the origin of life. Proc. Natl. Acad. Sci. USA 91: 1248-1250. Baltscheffsky H (1971). Inorganic pyrophosphate and the origin and evolution of biological energy transformation. In: Chemical evolution and the origin of life (Buvet R and Ponnamperuma C, eds.). North-Holland Biomedical Press, Amsterdam, 392-419. Baltscheffsky H (1977). Conversion of solar energy into energy-rich phosphate compounds. In: Living systems as energy converters (Buvet R, ed.). North-Holland Biomedical Press, Amsterdam, 199-207. Banks DA (1985). A fossil hydrothermal worm assemblage from the Tynagh lead-zinc deposit in Ireland. Nature 313: 128-131. Banks DA, Boyce AJ and Samson IM (2002). Constraints on the origins of fluids forming Irish Zn-Pb-Ba deposits: evidence from the composition of fluid inclusions. Econ. Geol. 97: 471-480. Barriga FJAS and Seahma Team (1998). Discovery of the Saldanha hydrothermal field on the famous segment of the MAR (36° 30' N). Eos. Trans. Am. Geophys. Union 79: 67. Baymann F, Lebrun E, Brugna M, Schoepp-Cothenet B, et al. (2003). The redox protein construction kit: pre-last universal common ancestor evolution of energy-conserving enzymes. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 358: 267-274. Berndt ME, Allen DE and Seyfried WE Jr (1996). Reduction of CO2 during serpentinization of olivine at 300°C and 500 bar. Geology 24: 351-354. Berry S (2002). The chemical basis of membrane bioenergetics. J. Mol. Evol. 54: 595-613. Bloch KE (1983). Sterol structure and membrane function. CRC Crit. Rev. Biochem. 14: 47-92. Bloch KE (1985). Cholesterol, evolution of structure and function. In: Biochemistry of lipids and membranes (Vance DE and Vance JE, eds.). Benjamin Cummings, New York, 1-24. Boyce AJ, Coleman ML and Russell MJ (1983). Formation of fossil hydrothermal chimneys and mounds from Silvermines, Ireland. Nature 306: 545-550. Butlerow A (1861). Formation sinthétique d´ une substance sucrée. C. R. Acad. Sci. 53: 145-147. Cairns-Smith AG (1982). Genetic takeover and the mineral origins of life. Cambridge University Press, Cambridge. Cody GD, Boctor NZ, Filley TR, Hazen RM, et al. (2000). Primordial carbonylated iron-sulfur compounds and the synthesis of pyruvate. Science 289: 1337-1340. Corliss JB, Baross JA and Hoffmann SE (1981). An hypothesis concerning the relationship between submarine hot springs and the origin of life on Earth. Oceanol. Acta 4: 59-69. Darwin F (1887). The life and letters of Charles Darwin. John Murray, London. Deamer DW (1997). The first living systems: a bioenergetic perspective. Microbiol. Mol. Biol. Rev. 61: 239-261. Deamer DW, Dworkin JP, Sandford SA, Bernstein MP, et al. (2002). The first cell membranes. Astrobiology 2: 371-381. Edmonds CG, Crain PF, Gupta R, Hashizume T, et al. (1991). Posttranscriptional modification of tRNA in thermophilic archaea (Archaebacteria). J. Bacteriol. 173: 3138-3148. Eigen M (1971). Selforganization of matter and the evolution of biological macromolecules. Naturwissenschaften 58: 465-523. Eigen M and Schuster P (1979). The hypercycle: a principle of natural self-organization, Springer-Verlag, Berlin. Forterre P (1995). Thermoreduction, a hypothesis for the origin of prokaryotes. C. R. Acad. Sci. 318: 415-422. Forterre P, Confalonieri F, Charbonnier F and Duguet M (1995). Speculations on the origin of life and thermophily: review of available information on reverse gyrase suggests that hyperthermophilic procaryotes are not so primitive. Origins Life Evol. Biosphere 25: 235-249. Gánti T (1971). Az élet princípuma (The principle of life). Gondolat, Budapest. Gánti T (1984a). Coupling of autocatalytic cycles as a possible explanation of chemical oscillators. Reac. Kinet. Catal. Lett. 24: 197-202. Gánti T (1984b). Chemoton theory. Vol. I. Theoretical foundation of fluid machineries original title (in Hungarian). OMIKK, Budapest. Gánti T (2002). On the early evolutionary origin of biological periodicity. Cell Biol. Int. Rep. 26: 729-735. Gánti T (2003). The principles of life. Oxford University Press, Eynshaw. German C and Parson LM (1998). Distributions of hydrothermal activity along the Mid-Atlantic ridge: interplay of magmatic and tectonic controls. Earth Planet. Sci. Lett. 160: 327-341. German CR, Parson LM and HEAT Scientific Team (1996). Hydrothermal exploration near the azores triple junction: tectonic control of venting at slow-spreading ridges? Earth Planet. Sci. Lett. 138: 93-104. Gracia E, Charlou JC, Radford-Knoery J and Parson LM (2000). Non-transform offsets along the Mid-Atlantic ridge south of the Azores (38°N-34°N): ultramafic exposures and hosting of hydrothermal vents. Earth Planet. Sci. Lett. 177: 89-103. Haldane JBS (1929). The origin of life. Ration. Annu. 148: 3-10. Haymon RM, Koski RA and Sinclair C (1984). Fossils of hydrothermal vent worms from cretaceous sulfide ores of the samail ophiolite. Oman. Sci. 223: 1407-1409. Heinen W and Lauwers AM (1996). Organic sulfur compounds resulting from the interaction of iron sulfide, hydrogen sulfide and carbon dioxide in an anaerobic aqueous environment. Origins Life Evol. Biosphere 26: 131-150. Huber C and Wachtershauser G (1997). Activated acetic acid by carbon fixation on (Fe, Ni)S under primordial conditions. Science 276: 245-247. Janecky DR and Seyfried WE Jr (1986). Hydrothermal serpentinization of peridotite within the ocean crust: experimental investigations of mineralogy and major element chemistry. GCA 50: 1357-1378. Kelley DS, Karson JA, Blackman DK, Fruh-Green GL, et al. (2001). An off-axis hydrothermal vent field near the Mid-Atlantic ridge at 30 degrees N. Nature 412: 145-149. King CC (1990). Did membrane electrochemistry precede translation? Origins Life Evol. Biosphere 20: 15-25. Koch AL (1984). Primeval cells: possible energy-generating and cell-division mechanisms. J. Mol. Evol. 21: 270-277. Koch AL and Schmidt TM (1991). The first cellular bioenergetic process: primitive generation of a proton-motive force. J. Mol. Evol. 33: 297-304. Larter RCL, Boyce AJ and Russell MJ (1981). Hydrothermal pyrite chimneys from the Ballynoe Baryte deposit, Silvermines, County Tipperary, Ireland. Miner. Depos. 16: 309-318. Leibniz GW (1705). Considerations sur les principes de vie et sur les natures plastiques par l’auteur de systéme de l’harmonie préétablie. J.E. Erdmann: God. Guil. Leibnitii Opera Philisophica, quae extant Latina Gallica Germanica omnia, 429-432 Berolini, 1840. Leibniz GW (1714). La Monadologie. J.E. Erdmann: God. Guil. Leibnitii Opera Philosophica, quae extant Latina Gallica Germanica omnia, 705-712 Berolini, 1840. Martin M and Russell MJ (2002). On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 358: 59-85. Miller SL (1953). A production of amino acids under possible primitive earth conditions. Science 117: 528-529. Miller SL (1955). Production of some organic compounds under possible primitive earth conditions. J. Am. Chem. Soc. 77: 2351-2361. Miller SL and Orgel LE (1974). The origins of life on the earth. Prentice Hall Inc., Englewood Cliffs. Miller SL and Bada JL (1988). Submarine hot springs and the origin of life. Nature 334: 609-611. Miller SL and Lazcano A (1995). The origin of life - did it occur at high temperatures? J. Mol. Evol. 41: 689-692. Morowitz HJ (1981). Phase separation, charge separation and biogenesis. Biosystems 14: 41-47. Morowitz HJ (1992). Beginnings of cellular life. Yale University Press, New Haven. Morowitz HJ, Heinz B and Deamer DW (1988). The chemical logic of a minimum protocell. Origins Life Evol. Biosphere 18: 281-287. Morowitz H, Peterson E and Chang S (1995). The synthesis of glutamic acid in the absence of enzymes: implications for biogenesis. Origins Life Evol. Biosphere 25: 395-399. Oparin AI (1924). The origin of life. In: Izd. Moskovshii. Rabochii, Moscow, Russia. English translation in J.D. Bernal. 1967. The origin of life (Weidenfeld and Nicolson, eds.). United Kingdom, London, 199-234. Oudin E and Constantinou G (1984). Black smoker chimney fragments in Cyprus sulfide deposit. Nature 308: 349-352. Rouse RC, Peacor DR and Freed RL (1988). Pyrophosphate groups in the structure of canaphite CaNa2P2O7 · 4H2O: first occurrence of condensed phosphate as a mineral. Am. Min. 73: 168-171. Russell MJ and Hall AJ (1997). The emergence of life from iron monosulphide bubbles at a submarine hydrothermal redox and pH front. J. Geol. Soc. 154: 377-402. Russell MJ, Hall AJ, Cairns-Smith AG and Braterman PS (1988). Submarine hot springs and the origin of life. Nature 336: 117. Russell MJ, Hall AJ and Mellersh AR (2003). On the dissipation of thermal and chemical energies on the early Earth: the onsets of hydrothermal convection, chemiosmosis, genetically regulated metabolism and oxygenic photosynthesis. In: Natural and laboratory simulated thermal geochemical processes (Ikan R, ed.). Kluwer Academic Publishers Group, Dordrecht, 325-388. Schopf JW (1983). Earth’s earliest biosphere: its origin and evolution. Princeton University Press, Princeton. Schrödinger E (1944). What is life? The physical aspect of the living cell. Cambridge University Press, New York. Schutz M, Brugna M, Lebrun E, Baymann F, et al. (2000). Early evolution of cytochrome bc complexes. J. Mol. Biol 300: 663-675. Shapiro R (1984). The improbability of prebiotic nucleic acid synthesis. Orig. Life Evol. Biosph. 14: 565-570. Shapiro R (1986). Origins: a skeptic’s guide to the creation of life on Earth. Heinemann, London. Shapiro R (1988). Prebiotic ribose synthesis: a critical analysis. Orig. Life Evol. Biosph. 18: 71-85. Shock EL and Schulte MD (1998). Organic synthesis during fluid mixing in hydrothermal systems. J. Geophys. Res. 103E: 28513-28527. Spiro TG (1997). Resonance raman results. Science 278: 21. Van Dover CI (2000). The ecology of deep-sea hydrothermal vents. Princeton University Press, Princeton. Wachtershauser G (1988a). Before enzymes and templates: theory of surface metabolism. Microbiol. Rev. 52: 452-484. Wachtershauser G (1988b). Pyrite formation, the first energy source for life: a hypothesis. Syst. Appl. Microbiol. 10: 207-210. Wachtershauser G (1990). Evolution of the first metabolic cycles. Proc. Natl. Acad. Sci. USA 87: 200-204. Wachtershauser G (1992). Groundworks for an evolutionary biochemistry: the iron-sulphur world. Prog. Biophys. Mol. Biol. 58: 85-201. Wachtershauser G (1994). Life in a ligand sphere. Proc. Natl. Acad. Sci. USA 91: 4283-4287. Wachtershauser G (2000). Origin of life. Life as we don’t know it. Science 289: 1307-1308. Weber AL (1987). The triose model: glyceraldehyde as a source of energy and monomers for prebiotic condensation reactions. Origins Life Evol. Biosphere 17: 107-119. Weber AL (1989). Model of early self-replication based on covalent complementarity for a copolymer of glycerate-3-phosphate and glycerol-3-phosphate. Origins Life Evol. Biosphere 19: 179-186. Weber AL and Hsu V (1990). Energy-rich glyceric acid oxygen esters: implications for the origin of glycolysis. Origins Life Evol. Biosphere 20: 145-150. Woese CR (1979). A proposal concerning the origin of life on the planet earth. J. Mol. Evol. 13: 95-101. |
|