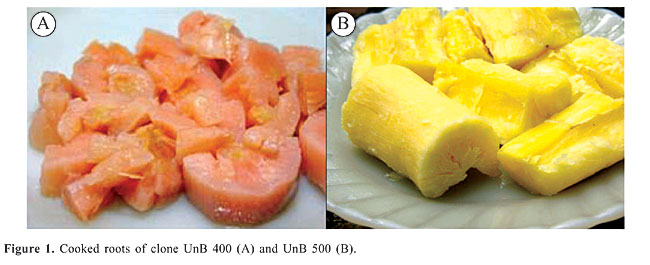
ABSTRACT. In Brazil, the center of cassava origin, cassava landraces have acquired through their domestication a large diversity in relation to many economic traits such as high content of carotenoids and excellent palatability among other characters. One of these clones, which has been grown by indigenous Brazilian farmers and is now being maintained in the University of Brasília gene bank, showed a high level of lycopene content (5 mg/kg viz. a viz. zero in common cultivars, and 12-20 mg/kg in tomato, a lycopene-rich vegetable). A second landrace called UnB 400 had a high content of b-carotene, which reached 4 mg/kg. Key words: Center of diversity, Landraces, Mutation accumulation, Carotenoids, Selection INTRODUCTION Cassava is the most important crop in the tropics and a staple food for more than 800 million people. Cassava is also the principal food for about 60 million people living in northeast Brazil. The majority of cassava clones grown and consumed in this region are known to be free of carotenoids, which leads to many health problems for inhabitants of this region. One of the interesting aspects is to screen indigenous clones for cultivars rich in carotenoids. This concept is based on the fact that crop landraces have accumulated, in their center of diversity, desirable mutations that were selected by indigenous farmers during their history of cultivation. The nutritive importance of carotenoids is attributed to its conversion to vitamin A, as in the case of b-carotene, and to its antioxidant property and ability to quench singlet oxygen as in the case of lycopene. Lycopene interacts with free radicals eliminating their deleterious effect. Various sources of morphological evidence (Nassar et al., 2005) have referred to the probability that some landraces maintained at our gene bank may be a source of carotenoids. These instances of morphological evidence include red root flesh and yellow color after cooking. For this reason two landraces, namely UnB 500 which has a red color and UnB 400 which has a yellow color after cooking, were analyzed spectrophotometrically. MATERIAL AND METHODS The landraces UnB 400 and 500 which are indigenous cassava clones grown in the Amazon and maintained in the University of Brasília’s living Manihot species collection were analyzed for lycopene content. Clone 400 has a gray stem, which is 1.5 m high, has large raised scars and 2-3 branched. Leaves have 5-7 lobes, and the leaf lobe is obvater, with the margins slightly sinuous, whereas the medium lobe is 14 to 16 cm in length. The leaf has a green petiole, and the young foliage is reddish. The inflorescence is a 5- to 8-cm glabrous panicle. Bracts and bracteoles are inconspicuous and caduceus. Flowers are monoecious showing pistillate flowers with basal opening, whereas the staminate apical opening occurs 3 weeks later. Fruits are green and winged and the roots are conical, with a rough, pink-brownish surface. The root flesh is creamy but turns yellow after cooking (Figure 1A). Clone 500 has a gray stem, stem is 1.5 m high, with large raised scars and 2-3 branches. Its leaves are 7-lobed, and the leaf lobe is linear, with the margins slightly sinuous, whereas the medium lobe shows 10 to 12 cm in length. The leaf has a green petiole, and the young foliage is reddish. The inflorescence is a 3- to 6-cm glabrous panicle. Bracts and bracteoles are inconspicuous and caduceus. Flowers are monoecious showing pistillate flowers with basal opening, whereas the staminate apical opening occurs 3 weeks later. Fruits are green and winged and the roots are conical, with a rough, pink-brownish surface. The root flesh is slightly red and turns dark red after cooking (Figure 1B).
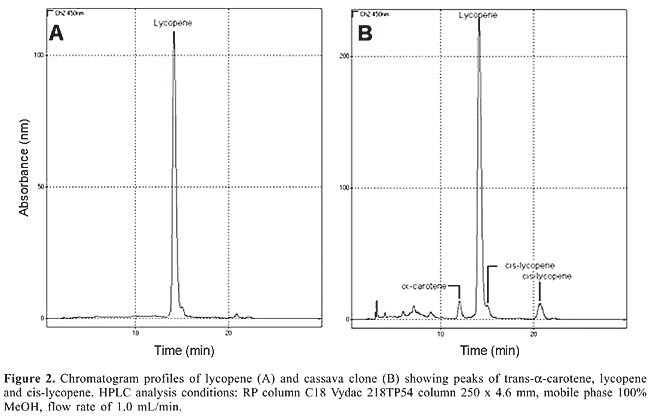
Extraction for lycopene analysis Ten grams of mature roots was extracted three times with acetone (5 mL per gram). The filtered acetone extract was mixed with petroleum ether and distilled water in a separation funnel. The aqueous fraction was discarded and the organic fraction was submitted to saponification. Saponification was preferred since it removes accompanying lipids. In the present study, the optimal conditions for mild saponification were achieved with 10% methanolic potassium hydroxide solution (100 mL) overnight at room temperature. After saponification, the aqueous fraction was discarded and the organic fraction was dried with anhydrous sodium sulfate. The organic fraction was evaporated to dryness at 30°C, resuspended in 1000 µL ethyl acetate and methanol (v/v; 50/50) and submitted to HPLC separation. Equipment Carotenoid analyses were performed with a Shimadzu LC-10A HPLC equipped with a photodiode array detector SPD MXA-10 and a Rheodyne injection valve with a 20-µL loop. The separation was carried out in a C18 Vydac 218TP54 column 250 x 4.6 mm ID (5-µm particle size) with 100% MeOH as mobile phase at a flow rate of 1 mL/min at a temperature of 15°C. The chromatograms were determined at maximum absorption (450 nm). The identification of carotenoids was achieved by retention time comparisons with those of the standard compounds and using the wavelength of maximal absorption (lmax) and the spectrum profile between 300 to 600 nm compared with data available in the literature (Krinsky, 1994). Quantification The calibration curves for lycopene were purchased from Sigma Inc., where lycopene was purified from tomato, while for trans-b-carotene (purchased from Sigma Inc.) and a-carotene (purified from alfafa) calibration curves were constructed using a minimum of three concentrations. All curves showed a good relationship between area and concentration, achieving a coefficient of determination (R2) of 0.96, 1.00 and 0.98, for lycopene, trans-b-carotene and trans-a-carotene, respectively. The cis-isomer of lycopene was quantified using the calibration curve of lycopene. RESULTS The chromatogram profiles of lycopene isolated from tomato and the cassava clone extract are shown in Figure 2A and B, respectively. Lycopene was shown to be the major carotenoid, although a-carotene and cis-lycopene were also found. The identification and characterization of the peaks are given in Table 1. Other carotenoids could not be detected in the cassava clone, which showed a concentration of 5 mg lycopene per gram of wet root weight.
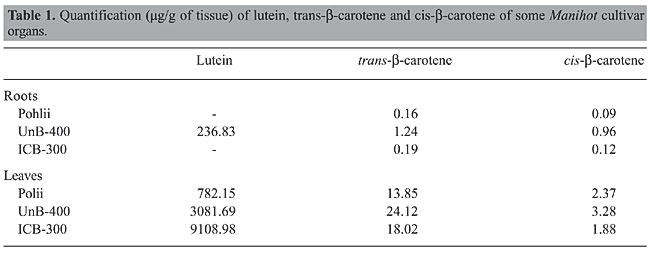
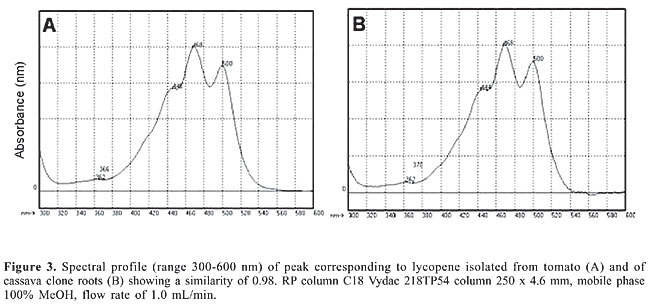
The retention time in HPLC system and the similarity of the spectral profile 300-600 nm in photo-diode array of 0.98 (Figure 3) confirm the presence of lycopene in this cassava clone. An interesting result was obtained when analyzing b-carotene in UnB 400. Trans-b-carotene reached 27.40 µg/g in this clone making it a source for this substance among indigent populations who depend on the daily consumption of cassava as an essential food. The root of this clone is creamy colored but turns yellow when cooked. Its palatability is excellent and almost free of fibers (Figure 1A,B).
DISCUSSION The most striking result of this research was the lycopene content in this cassava clone, which was not previously reported for this crop species to the best of the authors’ knowledge. This research provides a means of better understanding cassava domestication and further breeding by indigenous Amazon farmers. The clone is grown in the Amazon, and from there it was brought to the State of São Paulo, where it was further grown by a few farmers. This clone could have originated from a gene mutation that breaks the sequence of b-carotene formation, and then adopted by Amazon farmers who used it probably for rituals or cultural ceremonies. This clone forms few roots compared to other improved cultivars. However, increasing its root yield appears feasible by crossing with another clone possessing high combining ability for root yield. Lycopene occurs in tomato, guava, watermelon, and pink grapefruit and its consumption appears to be associated with reduced degenerative diseases. Other potential human health benefits include a possible role in the fight against digestive tract, breast and prostate cancers (Di Mascio et al., 1989; Krinsky, 1994). Other researchers have also emphasized lycopene’s protection against lung, stomach, and prostrate cancers (Stahl and Sies, 1996; Gerster, 1997; Sies and Stahl, 1998). Epidemiological studies have shown that high intake of vegetables containing lycopene is inversely associated with the incidence of certain types of cancer. For example, regular intake of tomato products has been inversely associated with the risk of cancer of the digestive tract. Lycopene is a precursor of b-carotene, whose synthesis includes an enzymatic cycle in the chain-end (Davies, 1976). The high lycopene level found in this cassava clone may indicate a disruption in the biosynthesis of b-carotene. The lycopene accumulation in this cultivar may therefore be the result of a deficiency in b-carotene synthesis due to a mutation. UnB 400 can be considered a good source for b-carotene in regions where cassava is the day-to-day principal food. b-carotene-like lycopene is an antioxidant, besides being a precursor of vitamin A, which is important in protecting against eye and skin diseases (Palozza and Krinsky, 1992; Handelman, 2001). ACKNOWLEDGMENTS We thank Conselho Nacional de Desenvolvimento Científico (CNPq) and Fundação de Aperfeiçoamento de Pessoal Superior (CAPES) for their financial support. We are grateful to the Brazilian Ministry of Environment for support to the senior author. REFERENCES Davies BH (1976). Carotenoids. In: Chemistry and biochemistry of plant pigments (Goodwin TW, ed.). Academic Press, London, 38-165. Di Mascio P, Kaiser S and Sies H (1989). Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 274: 532-538. Gerster HY (1997). The potential role of lycopene for human health. J. Am. Coll. Nutr. 16: 109-126. Handelman GJ (2001). The evolving role of carotenoids in human biochemistry. Nutrition 17: 818-822. Krinsky NI (1994). The biological properties of carotenoids. Pure Appl. Chem. 66: 1003-1010. Nassar NMA, Vizzotto CS, Silva HL, Schvartz CA, et al. (2005). Potentiality of cassava cultivars as a source of carotenoids. J. Food. Agric. Env. 3: 33-35. Palozza P and Krinsky NI (1992). Antioxidant effects of carotenoids in vivo and in vitro: an overview. Methods Enzymol. 213: 403-420. Sies H and Stahl W (1998). Lycopene: antioxidant and biological effects and its bioavailability in the human. Proc. Soc. Exp. Biol. Med. 218: 121-124. Stahl W and Sies H (1996). Lycopene: a biologically important carotenoid for humans? Arch. Biochem. Biophys. 336: 1-9. |
|