ABSTRACT. The purpose of the present study was to determine the effects of the steroidal plant hormone, 24-epibrassinolide (BL), on the mitotic index and growth of onion (Allium cepa) root tips. The classical Allium test was used to gather and quantify data on the rate of root growth, the stages of mitosis, and the number of mitoses in control and BL-treated groups of onions. Low doses of BL (0.005 ppm) nearly doubled the mean root length and the number of mitoses over that of controls. Intermediate doses of BL (0.05 ppm) also produced mean root lengths and number of mitoses that were significantly greater than those of the controls. The highest dose of BL (0.5 ppm) produced mean root lengths and number of mitoses that were less than control values, but the differences were not statistically significant. Examination of longitudinally sectioned root tips produced relatively similar results. This study confirms the suppositions of previous authors who have claimed that exogenously applied BL can increase the number of mitoses in plants, but failed to show cytogenetic data. This is the first report detailing the effects of BL on chromosomes and the cell cycle. Key words: Plant steroid, Brassinosteroids, Brassinolide, 24-Epibrassinolide, Mitotic index, Onion root tip chromosomes INTRODUCTION Discovery of brassinosteroids Mitchell et al. (1970) discovered that extracts from the pollen of the cruciferous plant (rape), Brassica napus L., produced an astonishing growth-promoting effect when applied exogenously to young pinto bean plants. After performing a bean second-internode assay, they claimed that the extract caused both increased cell elongation and cell division, but they did not perform classical mitotic chromosome counts. Grove et al. (1979) worked with 40 kg of bee-collected rape pollen and purified 4 mg of the active growth-promoting extract. They reported the isolation, structure determination and biological activity of the growth-promoting substance and called it “brassinolide” (BL). It was the first steroid hormone reported for the plant kingdom. They also carried out the bean second-internode bioassay and found that BL produced a nearly 200% increase in elongation of the internode compared to that of untreated controls. The dramatically increased internode elongation was accompanied by curvature, abnormal swelling and splitting of the internode. They hypothesized that these striking effects were attributed to increased cell division, although no mitotic counts were actually made. Structure of 24-epibrassinolide Grove et al. (1979) established the structure of BL by X-ray analysis of a single crystal of the purified extract. The structure of BL (Figure 1) is similar to that of animal steroid hormones, including ecdysone, progesterone and testosterone (Friedrichsen and Chory, 2001).
Discovery of other brassinosteroids Plant researchers have now discovered 40 plant steroids closely related to BL. These are collectively called brassinosteroids BRs. BRs are powerful hormones necessary for normal plant growth and development (Clouse and Sasse, 1998). There is evidence indicating that BRs are involved in the regulation of cell elongation, cell division, leaf bending, reproductive and vascular development, membrane polarization, proton pump, and modulation of stress (Kim et al., 2000). BRs are now considered to be as essential as the auxins, cytokinins, gibberellins, abscisic acid, and ethylene in the normal growth and development of plants (Clouse and Sasse, 1998). BRs exhibit striking structural similarities to animal steroids. The BL, 24-epibrassinolide, is believed to be the most powerful of the BRs. BRs are now classified as steroidal plant hormones. BRs are found in a variety of plant tissues throughout the plant kingdom. They have been identified in 27 higher plant families and three lower plant families (Bajguz and Tretyn, 2003). Purpose of the present study While some studies have claimed that BL promotes cell division, other studies have indicated that BL is highly inhibitory to cell division. However, none of these studies have sought to examine meristematic tissues in order to obtain quantitative data on the actual number of cells in various stages of mitosis following BL application. The purpose of the present study was to determine the effect of BL on mitosis by utilizing the classical Allium test (Fiskesjo, 1985). This involved growing onion roots in the presence of BL at various concentrations. While root length growth was important to this study, the primary purpose was to remove the growing onion root tip and to prepare microscopic slides of the meristematic region in order to gather quantitative data on the number of cells undergoing mitosis relative to the number of cells in interphase (mitotic index). It also allows data to be gathered on the number of cells in various phases of mitosis. This study sought to provide useful data on the effect of BL on mitosis. MATERIAL AND METHODS The Allium test was performed as described by Fiskesjo (1985). Forty-eight onion bulbs, approximately 2 cm in diameter, were selected for the study. Twelve onions were used for the control and each of the three experimental groups. The outer papery brown layer of each onion was peeled away and the dried basal root plate was removed with a sharp scalpel. Each of 48 test tubes were set up in test tube racks and filled with either spring water (controls) or spring water containing either 0.5, 0.05, or 0.005 ppm 24-epibrassinolide solution (experimental groups). The 24-epibrassinolide (BL) was obtained from Sigma Chemical Company, St. Louis, MO, USA, and has the formula 22R,23R,24R-2a,3a,22,23-tetrahydroxy-B-homo-7-oxa-5 a-ergostan-6-one. It has a minimum purity of 95%. A stock solution of 5 ppm BL was prepared by dissolving 0.005 g BL in 10 mL ethyl alcohol and adding this to 1 L spring water. The 0.05 and 0.005 ppm solutions were prepared by dilutions of the stock solution of BL with spring water. The control water stock solution was prepared by adding 10 mL ethyl alcohol to 1 L spring water. Each test tube was filled to the top with its respective solution and an onion was placed on top (Figure 2). A small amount of each of the above solutions was added to each respective test tube each day in order to replace that lost through evaporation. Stock solutions were stored in the refrigerator at 4°C. Of the 12 onions in each group, the 10 best developed onions of each series were chosen to complete the remainder of the experiment. On day 2 (48 h) after the initial experimental setup, microscope slides for mitotic studies were prepared using standard aceto-orcein staining (Fiskesjo, 1985). One root from five separate onions from each group (20 total roots) was removed, fixed (3 parts ethanol:1 part glacial acetic acid) and placed in a refrigerator overnight. The root tips were removed from the fixative, placed into 1 N HCl for 3 min, and transferred to aceto-orcein stain for 30 min to 1 h before squashing. The root tip was removed from the stain and rinsed in 45% glacial acetic acid for about 10 s. The root tip was placed on a microscope slide and 0.75 mm of the root tip was removed with a single-edge razor blade. Two drops of 45% glacial acetic acid were added and the root tip was squashed beneath a 22 x 40-mm cover glass using gentle, even thumb pressure. The monolayer preparation was ringed with clear fingernail polish and the meristematic region of the root was examined under a compound light microscope at 400X for stages of mitoses. The mitotic index was calculated by examining 400 cells of each root tip and by identifying each cell as being either in interphase, prophase, metaphase, anaphase, or telophase (2000 cells examined per group, a total of 8000 cells). The mitotic index is the percentage of cells in various stages of mitosis relative to the total number of cells examined. On day 4 (96 h), the root bundles were excised from all onions and were fixed in 3 parts ethanol:1 part glacial acetic acid and later washed and transferred to 95% ethyl alcohol. Each bundle root length was measured to the nearest millimeter using dial calipers. Individual roots were then cut from each bundle, counted, and measured to the nearest millimeter. Digital images were taken of the four groups of onions at the end of days 1 through 4. Figure 2A-H shows images of the onions and root length taken at the end of days 1 and 4. RESULTS Root growth The roots were completely removed from the onion bulb at the beginning of the experiment. By the end of day one (24 h), some root growth was apparent in all groups (Figure 2A-D). However, when compared with the control (Figure 2A), growth was obviously greater in the BL-treated onions, but was most evident in the experimental group treated with 0.005 ppm BL (Figure 2A-D). By the end of day four (96 h), root growth was obvious in all groups, but roots were visibly longer in experimental groups treated with BL when compared to the controls (Figure 2E-H).
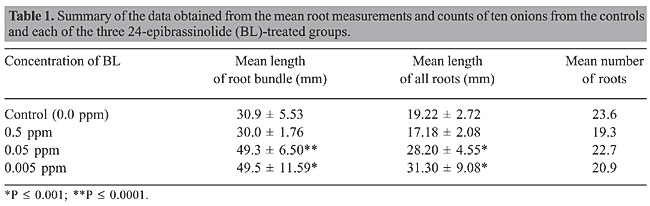
Effect of BL on the length and number of roots Table 1 provides a summary of data obtained from measuring root bundle lengths and the lengths of all individual roots and from counting the number of roots, from control and BL-treated onions. The mean length of the root bundles and the mean length of all roots grown in 0.5 ppm BL were not significantly different when compared to those of the controls. However, mean root bundle length and the mean length of all roots grown in 0.05 and 0.005 ppm BL were significantly greater when compared to those of the controls (P £ 0.0001 and P £ 0.001, respectively). Statistical analysis showed no significant difference between any of the groups relative to the mean number of roots.
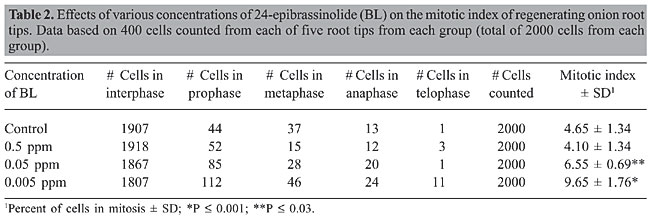
Effect of BL on the stages of mitosis and the mitotic index The number of cells in various stages of mitosis and the mitotic index (% of cells examined in mitosis) in controls and BL-treated root tips are shown in Table 2. Of 2000 cells counted, control onion root tips had 4.65% of the cells in various stages of mitosis. This number was nearly doubled to 9.65% in roots grown in 0.005 ppm BL. This was statistically significant from that of the controls (P £ 0.001). Those onion roots grown in 0.05 ppm BL also had a greater number of cells in mitosis than that of the controls (P £ 0.03). Those grown in 0.5 ppm BL did not show a mitotic index that was significantly different from that of the controls. While many differences are evident when comparing the various stages of mitosis in experimental groups with that of the controls, some values are striking and evident. First, the increase in mitotic index correlated with a dramatic increase in the number of prophases in the 0.005 and 0.05 ppm BL-treated cells. The number of prophases was nearly tripled in root tip cells treated with 0.005 ppm BL compared with that of the controls (112 vs 44). In fact, the number of cells in every stage of mitosis was increased in the 0.005 ppm BL-treated cells compared with that of the controls. The number of prophases was nearly doubled in those cells treated with 0.05 ppm BL (85 vs 44). Also evident, was the dramatic increase in the number of cells in telophase in the 0.005 ppm BL-treated cells as compared to that of the controls (11 vs 1). No statistically significant difference in mitotic index was seen between the controls and those root tip cells treated with 0.5 ppm.
DISCUSSION Effects of BL on root length growth Both promotive and inhibitory effects of BL on root growth of various plant species have been reported. Roddick and Guan (1991) found that BL had a direct inhibitory effect on seedling root growth. Hunter (2001) found that BL reduced root length in soybean. BL also inhibited the formation of adventitious roots in hypocotyls of dwarf bean, mung bean and cucumber (Adam and Marquardt, 1986). BL also inhibited root growth in seedlings of cress (Jones-Held et al., 1996), and in Arabidopsis thaliana (Clouse et al., 1993). Roddick (1994) found that several BRs inhibited the growth of excised tomato roots. Conversely, other researchers have found that BL has a promotive effect on root growth. Takatsuto et al. (1983) found that BL applied to the roots of radish or tomato seedlings promoted the elongation of the hypocotyls. Ronsch et al. (1993) found that the application of 28-homobrassinolide to cuttings of adult Norway spruce enhanced the formation of adventitious roots. Mussig et al. (2003) showed that low concentrations of BRs promote root elongation in Arabidopsis wild-type plants up to 50% and in a BR-deficient mutant up to 150%. Our study supported the finding of these latter authors. We found that onions grown in solutions of BL had a significantly greater root growth (elongation) at low doses of BL (0.005 and 0.05 ppm), but the highest dose (0.5 ppm) had no measurable effect (Table 1). Effects of BL on cell division Both promotive and inhibitory effects of BL on cell division have been reported (Worley and Mitchell, 1971; Grove et al., 1979; Sala and Sala, 1985; Roth et al., 1989; Clouse and Zurek, 1991; Nakajima et al., 1996; Oh and Clouse, 1998; Hu et al., 2000; Miyazawa et al., 2003). The earliest report of BL-mediated promotion of cell division was that of Worley and Mitchell (1971). They applied 10 µg BR to a young shoot internode of the pinto bean. The treated internodes grew longer and thicker than the controls. Histological sections showed that the increase in internode length was due to cell elongation in the basal part of the treated internode, while cells in the upper portion of the internode were stimulated to divide. However, the authors did not perform chromosomal or mitotic studies to relate these findings to the alleged increase in cell division. Rather, they showed that an average 4 mm length of the control internode contained 24 cortical cells while the same length from a BR-treated internode contained 48 cortical cells. Numerous other authors during the 1970’s and 1980’s reported that BL promoted cell division, but failed to conclusively substantiate their claim. More recently, the promotion of cell division by BL was reported in vitro in Chinese cabbage and in petunia protoplasts (Nakajima et al., 1996; Oh and Clouse, 1998). Conversely, Roth et al. (1989), working with Agrobacterium tumefaciens-transformed hormone-autonomous tobacco cells, showed that BRs were potent inhibitors of cell division. These contradictory results have proven to be enigmatic. Nakajima et al. (1996) and Hu et al. (2000) suggested that these confusing results on cell division could have their origin in the complex interaction of mitogenic factors with an unbalanced concentration or combination of phytohormones in the media. High BL concentrations are less effective in stimulating cell division, and often inhibitory (Hu et al., 2000). Additionally, the auxin and cytokinin status can affect the results when trying to determine the effect of BL on cell division (Oh and Clouse, 1998). The relationship between the effects of BL and auxin on cell proliferation is currently unresolved (Miyazawa et al., 2003). Effects of BL on cell division and cell-cycle-related gene expression Because contradictory results from previous studies have made the role of BRs in cell division unclear, Hu et al. (2000) used a cDNA array and identified genes that respond to BL in the det2 suspension culture of Arabidopsis and showed that BL induced the transcription of CycD3, a D-type cyclin gene which is used by cytokinin to activate cell division. Miyazawa et al. (2003) studied the effects of BRs on cell division using the tobacco (Nicotiana tabacum) BY-2 cell line. They found that BL promoted cell division only during the early phase of culture and in the absence of auxin (2,4-D). Furthermore, they confirmed the increased cell division by RNA gel blot analysis using cell-cycle-related gene probes. Effects of BL on cell division: the Allium test results in relation to previous studies Our findings herein using the Allium test support those authors who claim that BL promotes cell division, at least at lower doses. Our results also provide the first quantitative data relative to the effect of BL on the meristematic cells of the onion root tip. The data indicate that the number of cells undergoing mitosis may be doubled at lower doses (0.005 ppm) and that the number of cells in prophase and telophase may be more than tripled relative to that of the controls. We found this great increase in the number of cells in prophase and telophase within 48 h of exposure to BL. This supports the findings of Miyazawa et al. (2003) who reported that BL promoted cell division only during the early phase of culture. Because our BL-treated cells responded quickly with a significant increase in the number of prophases and telophases, this would indicate an alteration of the cell cycle with more cells entering and exiting mitosis than in the controls. Our findings of a greatly speeded up cell cycle in BL-treated cells tangentially support the findings of Hu et al. (2000) who showed that BL induces the transcription of cyclin genes which activate cell division. Because of the widespread presence of BL in many of our food plants and its powerful effects on the plant cell cycle, it would be of great interest to determine if BL has any similar effects on the cell cycle of humans. REFERENCE Adam G and Marquardt V (1986). Brassinosteroids. Phytochemistry 25: 1787-1799. Bajguz A and Tretyn A (2003). The chemical characteristic and distribution of brassinosteroids in plants. Phytochemistry 62: 1027-1046. Clouse SD and Zurek DM (1991). Molecular analysis of brassinolide action in plant growth and development. In: Brassinosteroids: chemistry, bioactivity, and application (Cutler HG, Yokota T and Adam G, eds.). American Chemical Society, Washington, 122-140. Clouse SD and Sasse JM (1998). Brassinosteroids: essential regulators of plant growth and development. Annu. Rev. Plant Physiol Plant Mol. Biol. 49: 427-451. Clouse SD, Hall AF, Langford M, McMorris TC, et al. (1993). Physiological and molecular effects of brassinosteroids on Arabidopsis thaliana. J. Plant Growth Regul. 12: 61-66. Fiskesjo G (1985). The Allium test as a standard in environmental monitoring. Hereditas 102: 99-112. Friedrichsen D and Chory J (2001). Steroid signaling in plants: from the cell surface to the nucleus. Bioessays 23: 1028-1036. Grove MD, Spencer GF, Rohwedder WK, Mandava NB, et al. (1979). Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 281: 216-217. Hu Y, Bao F and Li J (2000). Promotive effect of brassinosteroids on cell division involves a distinct CycD3-induction pathway in Arabidopsis. Plant J. 24: 693-701. Hunter WJ (2001). Influence of root-applied epibrassinolide and carbenoxolone on the nodulation and growth of soybean (Glycine max L.) seedlings. J. Agron. Crop Sci. 186: 217-221. Jones-Held S, VanDoren M and Lockwood T (1996). Brassinolide application of Lepidium sativum seeds and the effects on seedling growth. J. Plant Growth Regul. 15: 63-67. Kim S-K, Chang SC, Lee LJ, Chung W-S, et al. (2000). Involvement of brassinosteroids in the gravitropic response of primary root of maize. Plant Physiol. 123: 997-1004. Mitchell JW, Mandava N, Worley JF, Plimmer JR, et al. (1970). Brassins: a new family of plant hormones from rape pollen. Nature 225: 1065-1066. Miyazawa Y, Nakajima N, Abe T, Sakai A, et al. (2003). Activation of cell proliferation by brassinolide application in tobacco BY-2 cells: effects of brassinolide on cell multiplication, cell-cycle-related gene expression, and organellar DNA contents. J. Exp. Bot. 54: 2669-2678. Mussig C, Shin GH and Altmann T (2003). Brassinosteroids promote root growth in Arabidopsis. Plant Physiol 133: 1261-1271. Nakajima N, Shida A and Toyama S (1996). Effects of brassinosteroid on cell division and colony formation of Chinese cabbage mesophyll protoplasts. Jpn. J. Crop Sci. 65: 114-118. Oh MH and Clouse SD (1998). Brassinosteroid affects the rate of cell division in isolated leaf protoplasts of Petunia hybrida. Plant Cell Rep. 17: 921-924. Roddick JG (1994). Comparative root growth inhibitory of four brassinosteroids. Phytochemistry 37: 1227-1281. Roddick JG and Guan M (1991). Brassinosteroids and root development. In: Brassinosteroids: chemistry, bioactivity, and applications (Cutler HG, Yokota T and Adam G, eds.). American Chemical Society, Washington, 231-245. Ronsch H, Adam G, Matschke J and Schachler G (1993). Influence of (22S,23S)-homobrassinolide on rooting capacity and survival of adult Norway spruce cuttings. Tree Physiol 12: 71-80. Roth PS, Bach TJ and Thompson M J (1989). Brassinosteroids: potent inhibitors of transformed tobacco callus cultures. Plant Sci 59: 63-70. Sala C and Sala F (1985). Effect of brassinosteroid on cell division and enlargement in culture carrot (Daucus carota L.) cells. Plant Cell Rep 4: 144-147. Takatsuto S, Yazawa N, Ikekawa T, Takematsu T, et al. (1983). Structure-activity relationship of brassinosteroids. Phytochemistry 22: 2437-2441. Worley JF and Mitchell JR (1971). Growth responses induced by brassins (fatty plant hormones) in bean plants. J. Am. Soc. Hortic. Sci. 96: 270-273. |
|