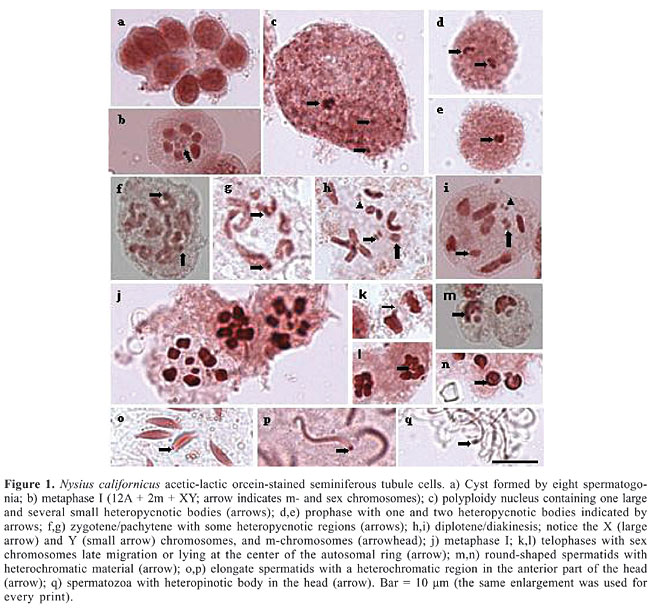
ABSTRACT. In Nysius californicus (family Lygaeidae, subfamily Orsillinae), a pest commonly known as the seed bug, the chromosome complement is 2n = 16 (12A + 2m + XY), testes are formed by seven seminiferous tubules covered by an orange-colored membrane, and spermatogenesis is cystic. At prophase, sex chromosomes are heteropycnotic and autosomes usually show a chiasma. At metaphase, sex chromosomes along with microchromosomes may be seen located at the center of a ring formed by the remaining autosomes. A characteristic specific of N. californicus was the presence of nucleolar material observed from the cystic cell to the completely differentiated spermatozoon. Variations in size, shape and location of the nucleolar material occur during this process, denoting a variable degree of activity in the different stages. Key words: Heteroptera, Lygaeidae, Nysius californicus, Karyotype, Heteropycnosis, Nucleolar corpuscles INTRODUCTION The members of the large and diverse family Lygaeidae (Heteroptera) are grain pests, commonly known as seed bugs (Schilling, 1829). According to several authors, this family is probably paraphyletic; some of its subfamilies are sister taxons to members of other Heteroptera groups from the families Berytidae, Colobathristidae, and Malcidae (Southwood and Leston, 1959; Stys, 1967). Thus, this family is of difficult taxonomic characterization and the complex relationship among its members is far from being established. Cytogenetic studies of the family Lygaeidae have been restricted to a few species (Ueshima and Ashlock, 1980). As reported for other Heteroptera, these species also have holocentric chromosomes, diverse sex chromosome systems, alternative sequences of meiotic reduction in autosomes and sex chromosomes, a pair of microchromosomes (m-chromosomes), B chromosomes, univalent chromosomes, and either chiasmatic or achiasmatic meiosis (Ueshima, 1979; Manna, 1984; Papeschi and Mola, 1990; González-Garcia et al., 1996; Grozeva and Nokkala, 1996; Suja et al., 2000; Nokkala and Nokkala, 2004). To date, simple XY/XX (74% of the species) and X0/XX (14.8%) or multiple sex chromosome systems (Xn0/XnXn, XnY/XnXn, XYn/XX) (10.3%) have been described, with multiple systems believed to have originated from the fragmentation of the X chromosome, or, less frequently, from the fragmentation of the Y chromosome. Another system, neo-XY, has been recently reported in three species (0.2%). The origin of this system very likely involves a subterminal insertion of the ancestor X chromosome into an autosome followed by a large inversion including part of the original X chromosome (Chickering and Bacorn, 1933; Schrader, 1940; Jande, 1959; Bressa et al., 1999; Nokkala et al., 2003). m-Chromosomes are known to be a characteristic of several Heteropteran families. First described in the species Caous ater (family Miridae) (Nokkala and Nokkala, 1986), m-chromosomes behave as univalents during diakinesis, and, as a rule, are negatively heteropycnotic over meiotic division (Grozeva and Nokkala, 2003). They do not pair at meiotic prophase, show “touch-and-go pairing” forming a pseudobivalent, and segregate at first meiotic division (Nokkala and Nokkala, 1983). Given that the families Reduviidae and Lygaeidae possess m-chromosomes, they have been considered to be very close from an evolutionary standpoint. In previous studies, diploid chromosome numbers ranging from 6 to 30 were observed in males of some species belonging to the family Lygaeidae (Grozeva and Kuznetsova, 1993). The smallest number was found in Artheneidae tenuicornis (Artheneinae) (Grozeva and Kuznetsova, 1990) and the largest in four species of the genus Cymus (Cyminae) (Ueshima and Ashlock, 1980). The most commonly found chromosome numbers are 2n = 14 and 2n = 16, with the latter appearing as a secondary form in many taxa. The purpose of the present study was to obtain further information about the chromosomes found in Lygaeidae species, as well as to analyze nucleolar characteristics and modifications that occur during meiosis in the seminiferous tubules of adult male Nysius californicus specimens. MATERIAL AND METHODS Specimens were collected from sunflowers (Helianthus annus) in São José do Rio Preto, SP, Brazil. Twenty individuals were used in the cytological preparations. In order to obtain seminiferous tubules, adult males were etherized, their wings were removed, abdominal sides were sectioned, and epidermis was separated. A drop of physiological solution was placed on the animal, testes were extracted and placed on slides (also in physiological solution), crushed and stained using the conventional lacto-acetic orcein technique, which stains DNA-associated proteins, or the Ag-NOR technique, which specifically stains nucleoli and nucleolar organizer regions due to its affinity with r-RNA-associated proteins (Howell and Black, 1980). Images were obtained with a Zeiss microscope using the image analyzer software AXI VISION. RESULTS AND DISCUSSION In Heteroptera, testes are characteristically formed by lobules arranged side by side and covered by a membrane. According to the literature, the number of lobules found in the family Lygaeidae is variable. Individuals with two, four, six, and seven lobules have been reported. However, seven is considered to be the ancestral number (Grozeva and Kuznetsova, 1992). The subfamilies Arthencinae, Oxycareninae and some Lygaeinae, such as Paranysius fraterculus, have the smallest number of lobules, just two (Grozeva and Kuznetsova, 1992). In the present study of N. californicus, seven seminiferous tubules or lobules, of almost equal size, arranged side by side and covered by a reddish membrane were observed, placing it among the species with ancestral characteristics. Heteroptera are also known to have cystic spermatogenesis, that is, germ cells are contained within an envelope, forming a simple layer of cells of mesodermal origin (Hannah-Alva, 1985 apud Schuetz, 1989). The cysts constitute isogenic groups of germ cells in which they develop into mature spermatozoa (Aiouaz, 1970 apud Schuetz, 1989). At the end of spermatogenesis, the cyst walls progressively degenerate and spermatozoa shofts are released into the collector duct. In the present study, eight spermatogonial cells were found inside N. californicus cysts (Figure 1a).
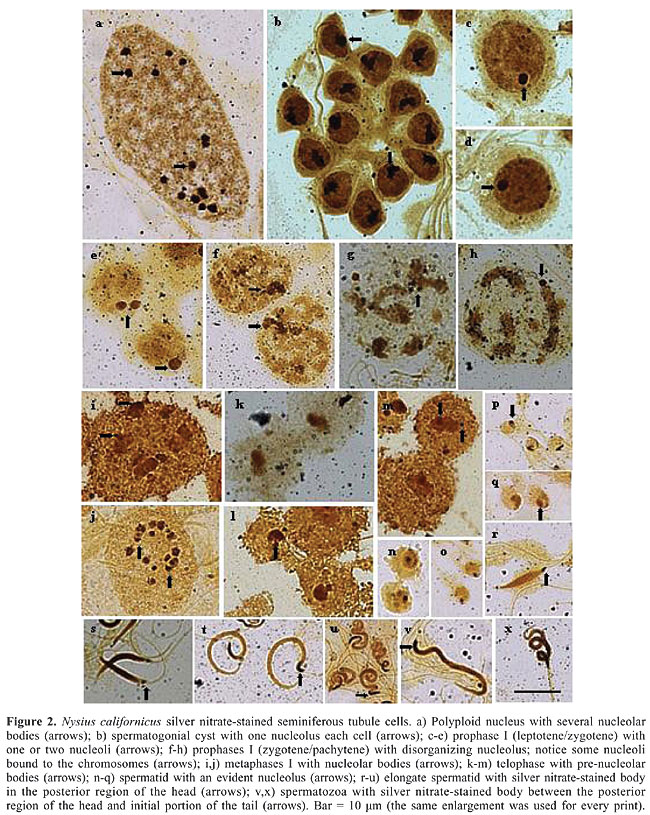
N. californicus showed a chromosome complement of 2n = 16 (12A + 2m + XY), in agreement with the results found to predominate in the tribe (Figure 1b). With regard to the number of chromosomes, in the Metrargini tribe, which includes the species N. californicus, seven genera and 31 species have been cytogenetically studied (Ueshima and Ashlock, 1980). Although karyotypes 2n = 16 predominated, some genera exhibited different karyotypes, such as Darwinysius with 2n = 14 (12 + XY) and Neseis with 2n = 18 (16 + XY). According to the literature, karyotype 2n = 18 derived from 2n = 16 by the duplication or fragmentation of one of the autosome pairs (Ueshima and Ashlock, 1980). Based on these grounds, it may be assumed that, in N. californicus, the chromosomal complement originated from individuals with 2n = 14, by the fragmentation or duplication of one of the autosomes. Fragmentation, usually considered more probable, gives rise to segments that could regularly migrate to the poles during anaphase and survive for many cell generations. Fragmentation is considered an important mechanism in the origin of increased chromosome number (Jacobs, 2004). On the basis of morphological characteristics, N. californicus has been considered a synonym for Xyonysius californicus (Emerson, 1988). Since the chromosomal complement 2n = 16 was reported in X. californicus, the finding of this chromosome number in Nysius may be evidence in support of the notion of synonymy. The present study showed that the sex chromosome system XY occurs in N. californicus. In males of the species Lygaeidae previously described, the sex chromosome system was XY or XO. About 80% of these species exhibit the XY type. Thus, the mechanism of sex determining in N. californicus is the predominating in the family. Ueshima and Ashlock (1980) suggested that, in Lygaeidae, the XY mechanism is the most primitive and XO derives from the loss of the Y chromosome during evolution. The species N. californicus (subfamily Orsilinae) displays two m-chromosomes, according to the rule in the family. According to Ueshima and Ashlock (1980), m-chromosomes are typical of all Lygaeidae subfamilies, except for Lygaeinae and Oxycareninae. Grozeva and Kuznetsova (1993), however, found m-chromosomes in 6 species of Oxycareninae. Thus, it is possible that only the most primitive subfamily, Lygaeinae, does not have m-chromosomes. Some details on chromosome morphology were also observed in the present study. One of them was the presence of more condensed regions, which are visible as heteropycnotic bodies or chromosome heteropycnotic regions in preparations stained with lacto-acetic orcein. In the polyploid nuclei of the nutritive cells the heteropycnotic bodies are variable in number and in size (Figure 1c), while at meiotic prophase I, the two heteropycnotic bodies observed probably correspond to the condensed X and Y chromosomes, which may be apart, but, most often, are found together at the periphery of the nucleus (Figure 1d,e). At zygotene/pachytene some autosomal regions are also heteropycnotic (Figure 1f,g). At diplotene/diakinesis, the X and Y sex chromosomes exhibit separate chromatids, two larger autosomes, frequently with a terminal chiasma, four smaller autosomes with telomeric association and two m-chromosomes (Figure 1h,i). At metaphase, depending on the cell orientation in the squash, autosomes are seen either aligned (side view) or arranged as a circle or ring (polar view) with the sex chromosomes and the m-chromosomes inside it. The m-chromosomes are located at the periphery of the ring forming a pseudobivalent (Figure 1j). At telophases, the sex chromosomes are seen either migrating lately or lying at the center of the autosomal ring (Figure 1k,l). A heteropycnotic body is observed again in the anterior region of the spermatid head, remaining in the same position up to the end of spermiogenesis and also in spermatozoa (Figure 1m-q). Other observations of Nysius made in this study, are related to the nucleolus. The analysis of silver nitrate-stained seminiferous tubules showed that the polyploid nuclei of their nutritive cells have several nucleolar bodies of different sizes (Figure 2a). The nucleoli of the spermatogonial cyst cells are large, found at the periphery of the nucleus, and exhibit quite variable morphology (Figure 2b). Cells at initial meiotic prophase are formed by one or two round-shaped nucleoli always located at the periphery of the nucleus (Figure 2c-e). Between pachytene and diplotene, nucleoli start to disorganize and lose their round shape (Figure 2f). Later at prophase, some smaller nucleolar bodies are distributed across the cytoplasm while others apparently associate with chromosomes (Figure 2g,h). Silver highlights are seen around the chromosomes during metaphase (Figure 2i,j), and at early telophase, the nucleolar body is reorganized (Figure 2k-m). Nucleoli are observed in all phases of spermiogenesis, varying its position: at the periphery of the nucleus when spermatids are round-shaped (Figure 2n-q), at the posterior region of elongated spermatids (Figure 2r-u), and in the posterior region of head in spermatozoa (Figure 2v,x). These observations indicate that nucleolar synthesis in N. californicus is different from that which predominates in the majority of arthropod species, involving nucleoli dissociation at diplotene or diakinesis. Therefore, in those species nucleoli cannot be detected by the Ag-NOR technique from metaphase to telephase I. Stained nucleolar bodies reappear when spermatids start to form, indicating the resumption of r-RNA transcriptional functions and disappear at the end of spermatid formation (Bressa et al., 2003).
Besides N. californicus, some species from other families and genera are exceptions to this pattern. For example in Triatoma brasiliensis and T. sordida (Heteroptera, Reduviidae) Ag-NOR-stained bodies remain observable up to metaphase I (Tavares and Azeredo-Oliveira, 1997). In Carlisis wahlbergi (Heteroptera, Coreidae) nucleolar bodies have been observed up to metaphase II (Fossey and Liebenberg, 1995), whereas in Acanthocoris sordidus (Heteroptera, Coreidae) and Coptosoma puntissimum (Heteroptera, Plataspidae) nucleoli have been detected in the metaphasic plates of primary and secondary spermatocytes (Yoshida, 1947). In Panstrongylus (Heteroptera) silver stains have been seen only in the initial spermatid (round-shaped), for they disappear as spermatids elongate (Tartarotti and Azeredo-Oliveira, 1999). This corroborates the hypothesis of post-meiotic genic reactivation to rRNA. This process has also been observed in mammals and other vertebrates (Hofgartner et al., 1979; Sumner, 1990). In the species under study, the presence of nucleolar material was seen from the cystic cell to the completely differentiated spermatozoon, suggesting the occurrence of protein synthesis throughout spermatogenesis. During this process, however, variations in size, shape and location occurred, indicating the dynamic functional involvement of the nucleolar material in the different stages of the process. Since the amount of nucleolar material has been reported to correlate with the biosynthetic activity of the cell, the size and number of nucleoli or nucleolar bodies, in N. californicus, may reflect metabolic differences between stages. The present results show that N. californicus shares some characteristics with other species from the same genus, but also shows different characteristics which reflect the wide variability that characterizes these organisms. ACKNOWLEDGMENTS The authors thank Dr. Luiz Antônio Alves Costa, Department of Entomology of the National Museum, Rio de Janeiro, RJ, Brazil, for the identification of the insects, and Dr. Sonia Maria Oliani, Department of Biology, IBILCE/UNESP, for the opportunity of capturing cell images, as well as FUNDUNESP and CNPq for their financial support. REFERENCES Bressa MJ, Papeschi AG, Mola LM and Larramendy ML (1999). Meiotic studies in Dysdercus Guerin Meneville 1831 (Heteroptera: Pyrrhocoridae). I. Neo-XY in Dysdercus albofasciatus Berg 1878, a new sex chromosome determining system in Heteroptera. Chromosome Res. 7: 503-508. Bressa MJ, Papeschi AG, Fumagalli E, van Doesburg PH, et al. (2003). Cytogenetic and nucleolar meiotic cycle analyses in Dysdercus imitator Blote, 1931 (Pyrrhocoridae, Heteroptera) from Argentina. Folia Biol. 51: 135-141. Chickering AM and Bacorn B (1933). Spermatogenesis in the Belatomatidae. IV. Multiple chromosomes in Lethocerus. Pap. Mich. Acad. Sci. Arts Lett. 17: 529-534. Emerson KC (1988). Entomology Museum. Entomology and plant pathology. Oklahoma State University. Checklist of the Hemiptera of Oklahoma. http://www.ento.okstate.edu/museum/hemiptera/Lygaeidae.htm. Accessed March 7, 2006. Fossey A and Liebenberg H (1995). Meiosis and nucleolar structures in the stink bug Carlisis wahlbergi Stal (Coreidae: Heteroptera). Cytobios 81: 7-15. Gonzalez-Garcia JM, Antonio C, Suja JA and Rufas JS (1996). Meiosis in holocentric chromosomes: kinetic activity is randomly restricted to the chromatid ends of sex univalents in Graphosoma italicum (Heteroptera). Chromosome Res. 4: 124-132. Grozeva SM and Kuznetsova VG (1990). Karyotypes and some structural properties of the reproductive system of bugs of the subfamily Artheneinae (Heteroptera, Pentatomomorpha, Lygaeidae). Entomol. Res. 69: 14-26. Grozeva SM and Kuznetsova VG (1992). The reproductive system of the some primitive families of pentatomomorphan bugs (Heteroptera). In: Advances in regulation of insect reproduction (Bennettova B, Gelbic I and Soldan T, eds.). Institute of Entomology, Czechoslovak Acadademy of Sciences, Prague, 97-102. Grozeva SM and Kuznetsova VG (1993). Notes on the karyotypes of some lygaeid bugs (Heteroptera, Pentatomomorpha, Lygaeidae). Folia Biol. 41: 65-75. Grozeva SM and Nokkala S (1996). Chromosomes and their meiotic behavior in two families of the primitive infraorder Dipsocoromorpha (Heteroptera). Hereditas 125: 31-36. Grozeva SM and Nokkala S (2003). C-heterochromatin and extra (B) chromosome distribution in six species of the genus Nabis (Heteroptera, Nabidae) with the modal male karyotype 2n = 16 + XY. Folia Biol. 51: 13-21. Hofgartner FJ, Schmid M, Krone W, Zenzes MT, et al. (1979). Pattern of activity of nucleolus organizers during spermatogenesis in mammals as analyzed by silver-staining. Chromosoma 71: 197-216. Howell WM and Black DA (1980). Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia 36: 1014-1015. Jacobs DH (2004). The evolution of a neo-XY1Y2 sex chromosome system by autosome sex chromosome fusion in Dundocoris nodulicarius Jacobs (Heteroptera: Aradidae: Carventinae). Chromosome Res. 12: 175-191. Jande SS (1959). Chromosome number and sex mechanism in twenty-seven species of Indian Heteroptera. Res. Bull. Panjab Univ. Sci. 10: 215-217. Manna GK (1984). Chromosomes in evolution in Heteroptera. In: Chromosomes in evolution of eukaryotic groups (Sharma AK and Sharma A, eds.). CRC Press, Boca Raton, 189-225. Nokkala S and Nokkala C (1983). Achiasmatic male meiosis in two species of Saldula (Saldidae, Hemiptera). Hereditas 99: 131-134. Nokkala S and Nokkala C (1986). Achiasmatic male meiosis of collochore type in the heteropteran family Miridae. Hereditas 105: 193-197. Nokkala S and Nokkala C (2004). Interaction of B chromosomes with A or B chromosomes in segregation in insects. Cytogenet. Genome Res. 106: 394-397. Nokkala S, Grozeva S, Kuznetsova V and Maryanska-Nadachowska A (2003). The origin of the achiasmatic XY sex chromosome system in Cacopsylla peregrina (Frst.) (Psylloidea, Homoptera). Genetica 119: 327-332. Papeschi AG and Mola LM (1990). Meiotic studies in Acanonicus hahni (Stal) (Coreidae, Heteroptera). I. Behaviour of univalents in desynaptic individuals. Genetica 80: 31-38. Schilling PS (1829). Hemiptera Heteroptera Silesiae systematice disposuit. Beitr. Entomol. 1: 34-92. Schrader F (1940). The formation of tetrads and the meiotic mitoses in the male of Rhytidolomia senilis Say (Hemiptera, Heteroptera). J. Morphol. 67: 123-141. Schuetz MJC (1989). Estudo da espermatogênese em Panstrongylus megistus Burmeister, 1835 (Hemiptera, Reduviidae). Doctoral thesis, Instituto de Biociências, USP, São Paulo. Southwood TRE and Leston D (1959). Land and water bugs of the British Isles. Frederick Warne and Co., London. Stys P (1967). Monograph of Malcinae, with reconsideration of morphology and phylogeny of related groups (Heteroptera, Malcidae). Acta Entomol. Mus. Nat. Pragae 37: 351-516. Suja JA, del Cerro AL, Page J, Rufas JS, et al. (2000). Meiotic sister chromatid cohesion in holocentric sex chromosomes of three heteropteran species is maintained in absence of axial elements. Chromosoma 109: 35-43. Sumner AT (1990). Nucleolar organizer (NORs). In: Chromosome banding (Thomas A, ed.). Unwin Hyman, London, 36-68. Tartarotti E and Azeredo-Oliveira MTV (1999). Patterns of nucleolar activity during spermatogenesis of two triatomines, Panstrongylus megistus and P. herreri. Caryologia 52: 177-184. Tavares MG and Azeredo-Oliveira MTV (1997). Pattern of nucleolar activity during spermatogenesis in triatomines (Heteroptera, Reduviidae) as analyzed by silver staining. Cytobios 89: 93-103. Ueshima N (1979). Hemiptera II: Heteroptera. Gebrüder Borntraeger, Berlin (Animal cytogenetics, Vol. 3: Insecta, 6). Ueshima N and Ashlock PD (1980). Cytotaxonomy of the Lygaeidae (Heteroptera). Univ. Kans. Sci. Bull. 51: 717-801. Yoshida T (1947). Unusual type of the nucleolus observed in a bug, Acanthocoris sordidus. J. Fac. Sci. Hokkaido Univ. (Zool.) 9: 243-249. |
|