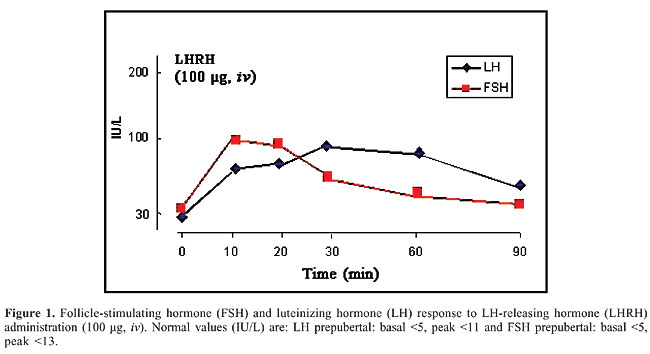
ABSTRACT. The aetiology of congenital bilateral anorchia is unknown. For many years there was speculation of an association between genetic factors and anorchia. We performed different tests in an anorchid boy, 2.5 years old, presented to us with micropenis and absence of both testes, in order to determine any possible factors contributing to the anorchia. Physical examination and hormonal, imaging, chromosomal, and molecular analyses of this case were performed. The basal FSH and LH levels were increased, and their increase in response to gonadotrophin-releasing hormone test was prolonged, while testosterone levels failed to increase after hCG administration. Ultrasonography of the pelvis and magnetic resonance of the abdomen were performed and failed to show any testicular tissue. Lastly, surgical exploration confirmed the absence of testicular structure. Chromosomal analysis revealed a normal male karyotype and molecular analysis did not reveal mutations or polymorphisms in the open reading frame of the SRY gene. Diagnostically, the lack of testosterone response to hCG stimulation is the hormonal hallmark of bilateral congenital anorchia. In addition, according to our case and previous studies, there is lack of association between genetic factors necessary for correct testicular descent and anorchia. Key words: Bilateral congenital anorchia, SRY gene, HCG test INTRODUCTION Bilateral congenital anorchia is defined as the complete absence of testicular tissue with a normal male karyotype and phenotype (Aynsley-Green et al., 1976). The true prevalence of this condition is unknown, because prospective studies have not been carried out. However, it is estimated to be approximately 0.5-1.0 cases per 20,000 males (Borrow and Gough, 1970). The pathogenesis of congenital anorchia has not been completely defined. The fetal testis must be present during the first 12 weeks of pregnancy for normal male genitalia to develop. It follows that an anorchid child with otherwise normal male external genitalia must have had, early in life, functional testes which have subsequently vanished (Bernasconi et al., 1992). The aim of the present study was to evaluate and discuss the clinical, hormonal, and imaging parameters in a patient affected with congenital bilateral anorchia and also to determine any possible relationship with genetic factors. PATIENT AND METHODS Hormonal analysis A patient aged 2.5 years old presented to us for evaluation of microphallus and bilateral absence of testes. Testosterone, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels were measured by radioimmunoassay. Normal values in prepubertal children are <0.8 nmol/L for testosterone, <5 mU/mL for LH and <5 mU/mL for FSH. Imaging analysis Ultrasonography of the pelvis was carried out using a scanner with 3.5-s acquisition times, and 1.5-mm thickness sections. Magnetic resonance imaging was performed by super-conductive magnetic resonance and superficial coil in transaxial sections. Chromosomal and molecular analysis Chromosomal analysis was performed with peripheral blood samples using standard procedures described previously (Iliopoulos et al., 2004). Fifty GTG-banded metaphases from PHA-stimulated peripheral blood lymphocytes were analyzed. DNA was extracted from peripheral blood lymphocytes using standard techniques. The entire open reading frame of the sex-determining gene SRY (sex-determining region, Y chromosome) was amplified by polymerase chain reaction and directly sequenced as described elsewhere (Lobaccaro et al., 1993). RESULTS A patient aged 2.5 years old, presented to us for evaluation of microphallus and bilateral absence of testes. He was a product of a non-consanguineous marriage. His antenatal history was uneventful and he was delivered by spontaneous vaginal delivery. At birth he was noted to have a small penis, with the urethral opening at the tip. The scrotum was small and empty. Family history was free and the patient’s only brother, aged 8 years old, has normal testes and normal penile length. Physical examination showed microphallus with the stretched penile length being lower than 2 SD below the mean for age. He had a hypoplastic scrotum without wrinkling and the testes were not palpable. Bone age was slightly retarded while ultrasonography and magnetic resonance imaging of the pelvis were negative. The serum basal level of testosterone was <0.5 nmol/L and did not change after the administration of human chorionic gonadotropin (hCG, 1000 IU im daily for three times). LH and FSH circulating levels were above the normal range and moreover, the administration of LHRH (gonadotropin-releasing hormone, GnRH) (100 µg, iv) induced a prolonged increase in these hormone levels (Figure 1).
Surgical exploration, which was performed in our patient because of his parents’ strong will, failed to show any testicular structure or Mullerian structures and the vas and vessels ended blindly in the abdomen. Chromosomal analysis using cultured peripheral lymphocytes revealed a normal male karyotype. Using the polymerase chain reaction, we amplified the open reading frame of the sex-determining region of the Y chromosome (SRY). No alterations, such as point mutations or polymorphisms of the SRY gene, were found. DISCUSSION The aetiology of bilateral congenital anorchia is not well understood. Various hypotheses have been advocated, such as an alteration in the gonadal vascularization due to an intrauterine torsion of both testes or an endocrinological disorder. On the basis of family occurrences of bilateral congenital anorchia a possible genetic aetiology has also been hypothesized (Huff et al., 1991). In particular, attention has been drawn to the sex-determining region of the Y chromosome, the SRY gene. However, we found no anomaly of the open reading frame sequence of the SRY genes, and these results are in agreement with other published studies (Lobaccaro et al., 1993; Parigi et al., 1999). A recent study demonstrated the absence of mutations in the SRY gene, in the INSL3 gene which is necessary for correct testicular descent and in the gene of its receptor (LGR8) in a cohort of 14 male patients with anorchia (Vinci et al., 2004). Although there are few studies concerning the involvement of genetic factors in patients with congenital anorchia, our results support the idea that genetic factors are probably not important in congenital anorchia. However, further genetic and molecular analyses are required and it is important to take advantage of very powerful tool, the DNA microarray analyses, in order to identify new genes and the molecular pathways related to congenital anorchia. The diagnosis of congenital anorchia should be suspected in a patient with male external genitalia, 46,XY karyotype and no detectable testes. In addition, a small phallus is a frequent clinical finding in anorchid patients (Bernasconi et al., 1992). The boy we present had all these characteristics of the disease. Clinical findings, however, are not enough to make the diagnosis, which must be confirmed by endocrinological evaluation and provocative tests, such as GnRH and hCG tests. The typical hormonal characteristics of congenital bilateral anorchia are elevated concentration of gonadotropins, especially FSH, low concentration of testosterone and lack of increase in plasma testosterone levels after hCG. In particular, this test plays a pivotal role in the diagnosis of bilateral congenital anorchia as is well demonstrated by many authors (Bablok et al., 1979; Jarow et al., 1986; Bernasconi et al., 1992; De Rosa et al., 1996). Moreover, the GnRH test induces a prolonged increase in FSH and LH levels (Aynsley-Green et al., 1976). In our patient, the hCG test demonstrated the lack of testosterone response and the GnRH test an abnormal increase in plasma FSH and LH levels, confirming the absence of both testes. With regard to the imaging study, ultrasonography is generally very useful because it is non-invasive and non-ionizing. However, it is actually difficult to differentiate between enlarged inguinal lymph nodes and testes and it is not definitive in the lumbar area where the cryptorchid testes are often situated. Magnetic resonance imaging may be the most innocuous and precise method, but it has the disadvantage of the long scanning time and the need for sedation (Fritzsche et al., 1987; De Rosa et al., 1996). According to previous studies (Belgorosky and Rivarola, 1982). surgical exploration can be resolutive. It must be carried out with great care considering the small dimensions of the organs, and it is especially helpful in unilateral anorchia (De Rosa et al., 1996). In our patient, surgical exploration, which was performed because his parents wanted it, failed to find any testicular elements or Mullerian structures, confirming once again the diagnosis of bilateral congenital anorchia. In conclusion, injection of hCG is, according to the opinion of many authors, the most useful test in the evaluation of anorchid patients (Levitt et al., 1978; De Rosa et al., 1996). In fact, in the case of failed increase of plasma testosterone levels, the imaging study and surgical exploration may be unnecessary. REFERENCES Aynsley-Green A, Zachmann M, Illig R, Rampini S, et al. (1976). Congenital bilateral anorchia: a clinical, endocrine and therapeutic evaluation of twenty-one cases. Clin. Endocrinol. 5: 381-391. Bablok L, Janczewski Z, Czaplicki M and Kwiatkowska Z (1979). Plasma testosterone levels before and after stimulation with HCG in anorchism. Int. Urol. Nephrol. 11: 57-60. Belgorosky A and Rivarola MA (1982). Sex hormone-binding globulin response to human chorionic gonadotropin stimulation in children with cryptorchidism, anorchia, male pseudohermaphroditism, and micropenis. J. Clin. Endocrinol. Metab. 54: 698-704. Bernasconi S, Ghizzoni L, Panza C, Volta C, et al. (1992). Congenital anorchia: natural history and treatment. Horm. Res. 37: 50-54. Borrow M and Gough MH (1970). Bilateral absence of testes. Lancet 14: 366-373. De Rosa M, Lupoli G, Mennitti M, Zarrilli S, et al. (1996). Congenital bilateral anorchia: clinical, hormonal and imaging study in 12 cases. Andrologia 28: 281-285. Fritzsche PJ, Hricak H, Kogan BA, Winkler ML, et al. (1987). Undescended testis: value of MR imaging. Radiology 164: 169-173. Huff DS, Wu HY, Snyder MCM III, Hadziselimovic F, et al. (1991). Evidence in favour of the mechanical (intrauterine torsion) theory over the endocrinopathy (cryptorchidism) theory in the pathogenesis of testicular agenesis. J. Urol. 146: 630-631. Iliopoulos D, Volakakis N, Tsiga A, Rousso I, et al. (2004). Description and molecular analysis of SRY and AR genes in a patient with 46,XY pure gonadal dysgenesis (Swyer syndrome). Ann. Genet. 47: 185-190. Jarow NP, Berkowitz GD, Migeon CJ, Gearhart JP, et al. (1986). Elevation of serum gonadotropins establishes the diagnosis of anorchism in prepubertal boys with bilateral cryptorchidism. J. Urol. 136: 277-279. Levitt SB, Kogan SJ, Schneider KM, Becker JM, et al. (1978). Endocrine tests in phenotypic children with bilateral impalpable testes can reliably predict “congenital” anorchism. Urology 11: 11-17. Lobaccaro JM, Medlej R, Berta P, Belon C, et al. (1993). PCR analysis and sequencing of the SRY sex determining gene in four patients with bilateral congenital anorchia. Clin. Endocrinol. 38: 197-201. Parigi G, Bardoni B, Avoltini V, Caputo M, et al. (1999). Is bilateral congenital anorchia genetically determined? Eur. J. Pediatr. Surg. 9: 312-315. Vinci G, Anjot MN, Trivin C, Lottmann H, et al. (2004). An analysis of the genetic factors involved in testicular descent in a cohort of 14 male patients with anorchia. J. Clin. Endocrinol. Metab. 89: 6282-6285. |
|