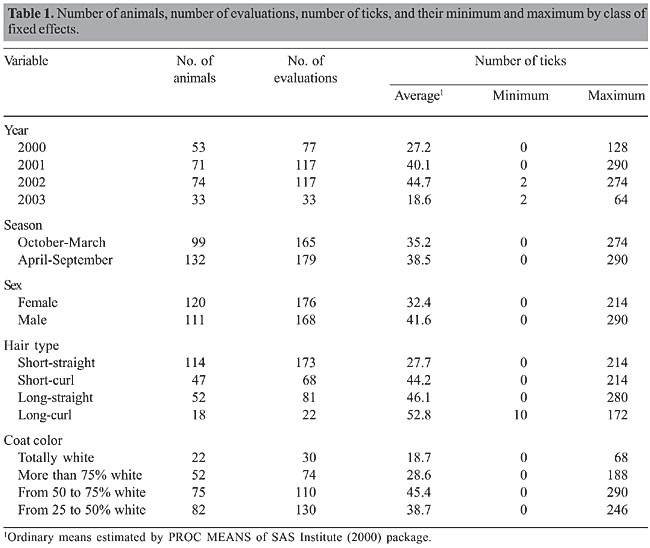
ABSTRACT. Losses caused by bovine tick burdens in tropical countries have a tremendous economic impact on production systems. Besides reducing production, this parasite can cause death in the most susceptible animals. The use of commercial acaricides has been the major method of control, but their misuse has led to tick resistance to many chemicals. More recently, vaccines have been used in some countries without solving the problem completely. An alternative could be the development of resistant animals and the use of genetic markers and candidate genes that could help with the enormous task of selecting resistant animals. The bovine lymphocyte antigen genes (BoLA) have been shown to be associated with some parasitic infestations and disease incidence. Thus, the objective of the present study was to determine the association of BoLA-DRB3.2 alleles with tick resistance in cattle. The study was conducted on 231 F2 (Gyr x Holstein) animals that were artificially infested with 10,000 tick larvae. Log of tick count +1 was used as the dependent variable in a mixed animal model with allele substitution effects in addition to fixed effects of year and season at tick count, sex of calves, age of animal at tick count, hair type (short-straight, short-curl, long-straight, and long-curl), coat color (white, >75% white, 50- 75% white, and 25-50% white), and additive genetic, permanent environmental and residual effects as random. Females showed fewer ticks than males. Animals with short-straight hair were more resistant to tick infestation than animals with long-curl hair, and animals with whiter coat color also had fewer ticks. An association between BoLA alleles and lower tick number was found for alleles DRB3.2 *18, *20 and *27 at the 5% significance level. Also, one allele (DRB3.2*16) showed an association at the 10% level. Allele *27 was the most frequent in the population (30.7%), followed by alleles *16 (10.8%), *20 (8.7%) and *18 (2.4%). These results suggest that BoLA-DRB3.2 alleles could be used to help in the selection of animals resistant to tick infestation. However, further studies involving a larger population of cattle in combination with other BoLA genes may help to understand the mechanisms of resistance to parasites. Key words: BoLA, Tick resistance, Bovine, Cattle, Candidate genes, Molecular markers INTRODUCTION Around a billion cattle, mainly located in tropical regions, could be afflicted by various tick species or by the diseases they transmit, which could lead to significant loss in production systems (Pegram et al., 1991). In these regions, infection by these parasites, besides reducing production, could cause the death of the more susceptible animals. In most Latin American countries the predominant species of bovine ticks is Boophilus microplus. In Brazil, which has the largest commercial bovine population in the world - around 170 million animals - losses caused by ticks and tick-related diseases are estimated to total US$800 million/year. Honer and Gomes (1990) estimated that cattle infected with ticks and worms could lose from 18 to 47 kg of live weight per year. In Australia, Frisch et al. (2000) estimated that an animal with an average of 40 ticks/day could lose weight equivalent to 20 kg/year. Also, they reported that animals infested with more than 200 ticks for a period of six weeks could die if not treated. The effect of tick infestation on milk production is also reported in the literature. In Brazil, Furlong et al. (1996) estimated a reduction of 23% in milk yield/day when crossbred Holstein-Zebu cows were infested with an average of 105 ticks. Also, Teodoro et al. (1998) reported a reduction of 529 kg (26%) of milk/lactation in Holstein cows untreated with acaricides. In Australia, Holstein cows of high production were submitted to an increasing burden of ticks every week for a total period of 105 days. In the last week, these cows showed a reduction of 2.86 kg of milk/day and 10.6 kg of live weight (Jonsson et al., 1998). For many years, tick control has been mostly based on the use of acaricides; however, their misuse has given rise to ticks resistant to pesticides and also increased environmental contamination. Vaccines have been used in some countries without solving the problem completely (Labarta et al., 1996; Frisch, 1999). Another alternative could be the use of resistant animals. However, the identification of resistant animals is costly and time consuming. The identification of molecular markers associated with tick resistance in cattle could be used in marker-assisted selection. The bovine lymphocyte antigen complex (BoLA) has been studied extensively for the past 20 years because of its importance in host immunity (Spooner et al., 1978). The Class I and Class II BoLA genes encode proteins mediating antigen presentation (Anderson, 1990; Teale et al., 1991). Studies have demonstrated associations between BoLA alleles and disease incidence. The Class I and Class II BoLA genes have been associated with the incidence of mastitis (Mejdell et al., 1994; Dietz et al., 1997; Kelm et al., 1997; Sharif et al., 1998), bovine leukemia virus infection (Roth and Kaeberle, 1981; Xu et al., 1993; Skow et al., 1998), chronic posterior spinal paresis (Park et al., 1993) and parasitic load (Stear et al., 1988). Investigations relating BoLA genes and tick resistance are rare. Stear et al. (1990) studied the relationship between ectoparasites (B. microplus ticks) and BoLA Class I antigens and showed that animals with the BoLA antigens W6.1 and W7 had significantly fewer ticks than animals lacking these antigens. Martinez et al. (2004) in a preliminary study, found a putative association between tick count and alleles *10 and *42 (P < 0.1) of the BoLA-DRB3.2 gene. They also reported a relationship between warble count and alleles *31 (P < 0.05), *42 (P < 0.10) and *51 (P < 0.05) in the same population. The objective of the present study was to determine the association of BoLA-DRB3.2 alleles with tick resistance in an F2 crossbred Holstein-Gyr population. MATERIAL AND METHODS Animals A total of 344 infestations in 231 F2 animals were studied to assess tick resistance. The F2 population was produced by crossing F1 females (50% Gyr: 50% Holstein) with F1 sires of the same genetic composition. The F1 cattle were generated by embryo transfer in 78 Gyr cows mated with four Holstein sires resulting in a total of 150 F1 animals (males and females). Of those, only 4 F1 sires were chosen according to their performance to mate with 68 F1 females, avoiding relationships among sires and cows. All F2 animals were raised together on an experimental farm located in Valença, State of Rio de Janeiro, Brazil, in a hilly region at an altitude between 200 and 400 m above sea level. The climate corresponds to Cwa of Koppen’s classification (Koppen and Geiger, 1936) (mild, dry winter, hot summer), with the dry season extending from April to September (Teodoro and Madalena, 2003). The experimental calves were artificially reared on approximately 4 L of whole milk/day and housed individually up to 8 weeks of age in an area free of ticks. From this age up to 6 months, they were kept in paddocks of Cynodon dactylon L, being fed up to 2 kg/head/day of 18% crude protein ration plus chopped elephant grass (Penissetum purpeream, Schumach). In these paddocks they started to have contact with ticks. Thereafter, they were allow to graze on pastures of predominantly Brachiaria decumbens (Stapf.) supplemented with chopped elephant grass plus 1 kg/head/day of concentrate in the dry season and periods of pasture shortage. Minerals were made available in the paddocks for all animals. During the period from birth to 10 to 14 months of age the F2 animals were not treated with any product to control ticks. Animals were studied in contemporary groups with age ranging from 10 to 14 months. To determine tick resistance, each F2 animal was artificially infested with approximately 10,000 tick larvae by placing them in the “dorsal-lumbar” region of the animals. Animals were kept tied up for 30 min to prevent grooming and to allow the larvae to spread to all regions of the body. Afterward, they were kept on pastures for 21 days when the tick count was conducted. All engorged female ticks 4.5 to 8.0 mm in length were counted on only one side of the animal, and the count was then multiplied by two. Infestations were carried out during the spring, summer and fall seasons, allowing some animals to be studied in two different seasons. DNA genotyping Blood samples were taken from all F2 animals to extract DNA to genotype for the BoLA-DRB3.2 alleles. DNA samples were obtained using a phenol-chloroform and proteinase K procedure. DNA quality and concentration were determined by UV spectroscopy. Amplification of the BoLA-DRB3.2 alleles was accomplished by a nested PCR procedure as described by van Eijk et al. (1992). The primers HLO30 (5´-ATCCTCTCTCTGCAGCACATTTCC-3´) and HLO31 (5´-TTAAATTCGCGCTCACCTCGCCGCT-3´) were used for the first amplification. The primers HLO30 and HLO32 (5´-TCGCCGCTGCCACAGT-3´) were used in the second amplification to increase PCR specificity and to decrease the presence of heteroduplexes. The HLO32 primer consists entirely of nucleotides from the 3’ end of exon 2 and has 8 nucleotides overlapping with primer the 3’ end of HLO31. The first amplification reaction consisted of 10 cycles of 94°C (60 s), 60°C (120 s) and 72°C (60 s). The PCR mixture contained 12.5 ng of template DNA, one unit of Taq polymerase, 100 µM of dNTPs, 0.2 µM of each primer, 10 mM Tris-HCl, pH 8.0, 2.5 mM MgCl2, and 50 mM KCl. The second amplification reaction mixture consisted of 30 cycles of 94°C (60 s), 65°C (30 s), using 1.4 µL from the first amplification reaction as DNA template in a final volume of 35 µL with one unit of Taq polymerase, 100 µM of dNTPs, 0.2 µM of each primer, 10 mM Tris-HCl, pH 8.0, 2.5 mM MgCl2, and 50 mM KCl. PCRs were carried out using the GeneAmp PCR System 9600 (Applied Biosystems). The PCR products generated in the second amplification reaction were visualized by loading 5 µL onto native polyacrylamide gels (5%, 500 volts, 1 h) to standardize DNA quantity as well as to detect amplification problems. Afterward, 10 µL of the second amplification reaction was digested for 2 h at 37°C with 5 units of RsaI and HaeIII restriction enzymes and for 2 h at 60°C with the enzyme BstyI in a final volume of 15 µL. Digested fragments were loaded onto native polyacrylamide gels (12%, 800 volts, 3 h) using 35-cm tall plates to assure perfect separation of the alleles. Gels were silver-stained and fragments were scored using 10- and 25-bp ladders. Fragment nomenclature followed van Eijk et al. (1992), BoLA Workshop Nomenclature Web Site (http://www.projects.roslin.ac.uk/bola/wk92b.html) and Maillard et al. (1999). Statistical analysis Allele frequencies of the BoLA-DRB3.2 gene for the F2 animals were calculated by direct count. Analysis of the data was performed by the following animal mixed model: Y = Xh + Za + Zp + Mm + E where: Y is the vector of log count +1; X and Z are matrix of zeros and ones related to the fixed and random effects; h, a, p, and E are the solution vectors to the fixed, additive genetic, permanent environmental, and residual effects, respectively; m is a vector that includes the regression effects of allele substitution of the BoLA gene, and M is a matrix of 0, 1 or 2 showing the number of copies present in each animal. Fixed effect includes year and season at counting, age of animals at counting, sex, hair type, and coat color. Additive genetic, permanent environment and residual effects were assumed to be randomly distributed with zero means and variances s2a, s2p and s2e, respectively. A complete relationship matrix with parents and grand-parents of the F2 animals were used. Analysis was carried out using the PROC MIXED of the SAS Institute (2002) package. Data distribution and variable description are given in Table 1.
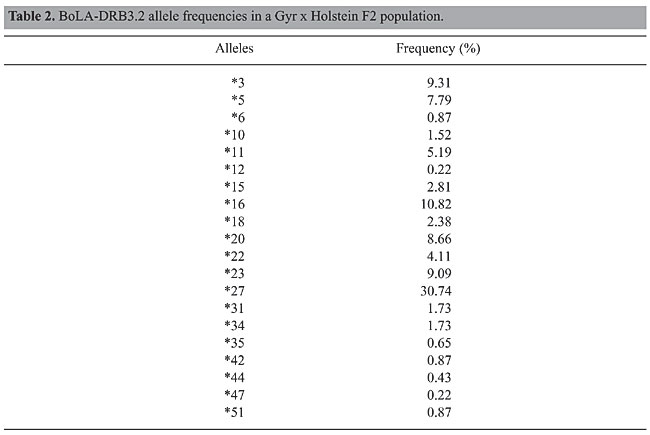
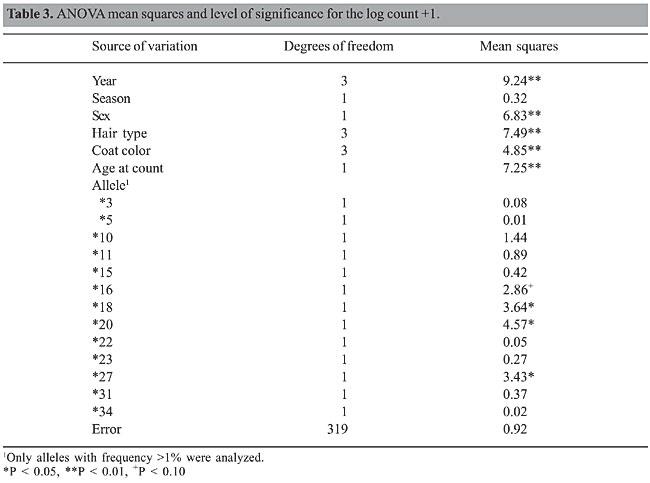
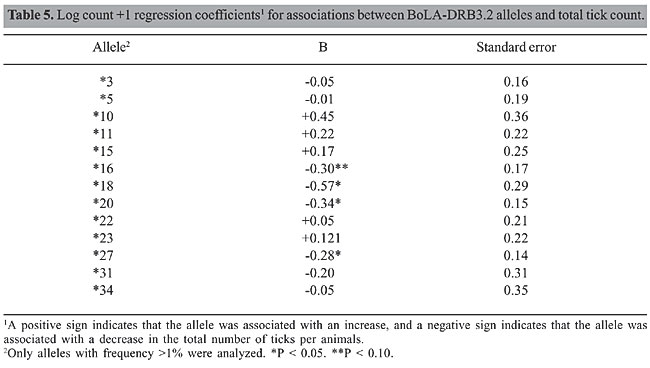
RESULTS Twenty BoLA-DRB3.2 alleles were found in the F2 population used in this study (Table 2). The most frequent one was allele *27 (30.74%), followed by alleles *16 (10.82%), *3 (9.31%), *23 (9.09%), and *20 (8.66%). Some of them showed very low frequencies (alleles *6, *12, *35, *42, *44, *47, and *51) and were eliminated from association studies. In Table 3, the analysis of variance (ANOVA) showed high level of significance (P < 0.01) for all effects except for the season at counting. The non-significant effect of season may be due to the fact that animals were artificially infested, and therefore, the level of tick infestation was independent of climate conditions during the year. Also, three alleles (*18, *20 and *27) showed significant correlation with decreased number of ticks per animal at the 5% level, and allele *16 showed significance at the 10% level.
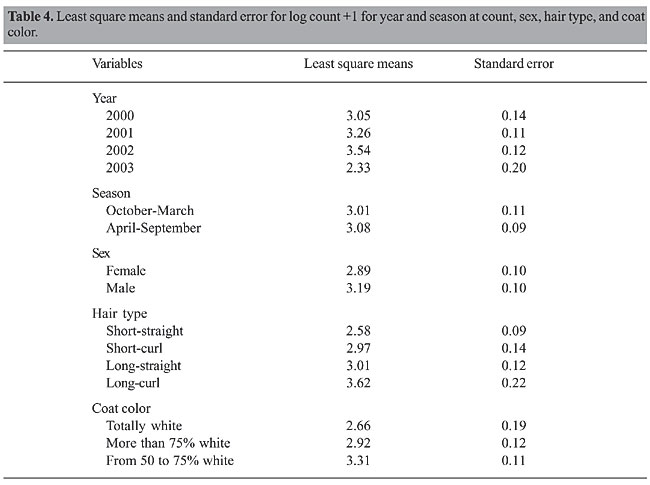
The least square means for the log count +1 (Table 4) indicated that males had more ticks than females. Some authors have observed the same sex effect and have suggested that it could be due to hormone differences (Stear et al., 1990; Veríssimo et al., 1997). Similarly, hair type and coat color had a significant effect in the log count of ticks per animal. There was a great variation in these effects, from 2.58 to 3.62 and from 2.66 to 3.29 for hair type and coat color, respectively. Animals with short-straight hair type showed fewer ticks than all the others. A possible explanation is that ticks may have more difficulty in attaching to this type of hair and that it is easier for the animal to groom itself (Veríssimo et al., 1996). Also, animals with a whiter coat had fewer ticks than darker coat animals. Since ticks are dark-colored, one explanation is that in darker animals, they would be protected against predators such as birds. To our knowledge similar results have not been previously published. We investigated the possibility of an interaction between hair type and coat color that could explain this effect, but the results were not significant.
The association between BoLA alleles and total tick count measured as the log count +1 regression coefficient is presented in Table 5. The results indicated a significant (P < 0.05) decrease in the total number of ticks when alleles *18, *20 or *27 were present. Also, in the presence of allele *16, there was a near-significant trend (P < 0.10) toward a decreased number of ticks/animal. Allele *18 had the greatest effect (-0.57) in decreasing the log count of ticks/animal. Although, allele *27 had a smaller effect (-0.28) compared with all the others, it is important to note that it was the most frequent in the population (30.74%) (Table 2). Selection of animals with allele *27 could lead to a rapid response, resulting in a decreasing number of ticks per animal as their progenies become increasingly resistant. Similarly, selecting parents possessing alleles *18, *20 or *27 for future generations should increase the stock’s resistance to tick infestation.
DISCUSSION Although selection for moderately to highly heritable traits such as production and carcass traits has been successful in cattle, attempts to decrease health problems using genetic selection have only achieved moderate success (Lie and Solbu, 1981; Stear et al., 1988; Frisch et al., 2000). In dairy cattle, selection for improved health has been hampered by low heritabilities among both direct and indirect health measures (Emanuelson et al., 1988). In poultry, selection based on MHC type has been successful in reducing the incidence of some specific diseases, and the potential for MHC-assisted selection has been demonstrated in a variety of other species (Warner et al., 1987). Such a marker system for disease susceptibility and parasite resistance could greatly facilitate selection for improved general health in cattle. In addition, marker information could be useful when selecting among animals of equivalent ancestor merit, such as selection among full-sib bulls in a MOET program. The results obtained in this study, along with those from other authors (Stear et al., 1990; Weigel et al., 1990; Dietz et al., 1997; Martinez et al., 2004), confirm the potential of BoLA alleles as molecular markers for health and production traits in cattle. However, particular allelic effects should not yet be considered conclusive. Another major study evaluating the association between tick resistance and BoLA alleles was conducted in Australia (Stear et al., 1984, 1989, 1990). It was reported that calves with BoLA antigen W6.1 or W7 clearly had fewer mature B. microplus larvae compared to calves lacking these antigens. Our results also indicate a reduction in tick number/animal when the BoLA-DRB3.2 alleles *18, *20, or *27 were present. Although our results with Class II BoLA cannot be directly compared with the Australian Class I BoLA results, they clearly show that the BoLA complex plays some role in the animals’ general health system making some animals more resistant to tick infestation than others. Some theoretical considerations suggest that Class II gene products may be more important in the expression of resistance than Class I gene products. Immediate hypersensitivity reactions at the site of tick attachment stimulate the removal of ticks by grooming. Animals prevented from grooming show a transient increase in tick numbers (Willadsen, 1980). Studies in humans suggest that Class II genes are more important than Class I genes in the regulation of the immediate hypersensitivity reaction (Freidhoft et al., 1988). Several authors (Kemp et al., 1971; Allen, 1973; Bagnall and Double, 1975) attribute the rejection of ticks by their hosts to the combined action of basophils, eosinophils, T lymphocytes, and IgG since such cells and particularly basophils predominate in the inflammatory reaction. However, basophils are recruited from the bone marrow, via the blood stream, to be activated locally at the point of the tick’s attachment to the host’s skin. These cells require a time interval of about 6 h, which is the minimum period elapsed before they are detected in the inflammatory infiltrate (Moraes et al., 1992). On the other hand, mast cells reside in the dermis and their degranulation can be triggered by the combined mechanical trauma caused by the tick’s mouth pieces and its injected saliva. In Zebuine hosts, undamaged skin has been found to harbor more than twice as many mast cells per surface unit than in the Taurine’s uninjured integument (Moraes et al., 1992). The degranulation of these cells liberates several pharmacologically active substances such as histamine among others which cause intense pruritus. This teases the animals into self-licking, becoming the main mechanism by which they get rid of most tick larvae. Moraes et al. (1992) reported that the self-cleaning activity through self-licking starts at half an hour to two hours after tick infestation. Therefore, this reaction begins long before basophils appear in the blood stream (about 6 h) after the tick stimulus elicited by the parasite’s initial bites. The evidence found by Moraes et al. (1992) of a much higher number of mast cells in the uninjured skin of Zebuines as compared to the number of these cells in the uninjured skin of taurine animals may explain - at least in part - the higher efficiency at which Zebuines get rid of their ticks when compared to Taurine animals. The immediate hypersensitivity reaction is mediated by IgG and IgE, and is associated with an increase in vascular permeability and corresponding edema due to local mast cell degranulation. In fact, mast cells can be seen in significant numbers at Rhipicephalus sanguineus feeding sites on dogs (Szabó and Bechara, 1999). These mast cells are probably coated with IgE or IgG following the first exposure to ticks, and may degranulate upon challenge with tick antigens. Although there are no results directly associating the BoLA complex with the number of mast cells, there is some indication of an association between the BoLA-DRB3.2 alleles *11, *24, *12, *3, and *28 and increased serum IgG2 concentration, and between allele *26 and decreased serum IgG2 (Dietz et al., 1997). Further studies involving a larger population of cattle in combination with other markers in the BoLA region may help to strengthen the interaction between the BoLA complex and traits of innate and adaptive immunity, and may also help to unravel the mechanisms of resistance to parasitic infections. ACKNOWLEDGMENTS We thank ABCGIL (Brazilian Gyr Cattle Association) for the Gyr blood samples. C.S. Nascimento was the recipient of a scholarship from FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais). Research supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and PRODETAB (Projeto de Apoio ao Desenvolvimento de Tecnologias Agropecuárias para o Brasil). REFERENCES Allen JR (1973). Tick resistance: basophils in skin reactions of resistant guinea pigs. Int. J. Parasitol. 3: 195-200. Anderson L (1990). Major histocompatibility genes in cattle and their significance for immune response and disease susceptibility. In: Genome analysis in domestic animals (Geldermann H and Ellendorff F, eds.). VCH Verlagsgesellschaft mbH, Weinheim, Germany, 213. Bagnall GG and Double BM (1975). The Australian paralysis ticks Ixodes holayclus. Aust. Vet. J. 51: 159-160. Dietz AB, Detilleux JC, Freeman AE, Kelley DH, et al. (1997). Genetic association of bovine lymphocyte antigen DRB3 alleles with immunological traits of Holstein cattle. J. Dairy Sci. 80: 400-405. Emanuelson U, Danell B and Philipsson J (1988). Genetic parameters for clinical mastitis, somatic cell counts, and milk production estimated by multiple-trait restricted maximum likelihood. J. Dairy Sci. 71: 467-476. Freidhoff LR, Ehrlich-Kautzky E, Meyers DA, Ansari AA, et al. (1988). Association of HLA-DR3 with human immune response to Lol p I and Lol p II allergens in allergic subjects. Tissue Antigens 31: 211-219. Frisch JE (1999). Towards a permanent solution for controlling cattle ticks. Int. J. Parasitol. 29: 57-71. Frisch JE, O’Neill CJ and Kelly MJ (2000). Using genetics to control cattle parasites - the Rockhampton experience. Int. J. Parasitol. 30: 253-264. Furlong J, Derez F, Matos LL and Balbi MV (1996). The effect of cattle tick Boophilus microplus (Acari: Ixodidal) infestation on feed intake and milk yield of Holstein x ZebuZebu crossbred cows. Proceedings of the 15 Honer MR and Gomes A (1990). O manejo integrado de mosca dos chifres, berne e carrapatos em gado de corte. Embrapa-CNPGC, Brazil. Circular Técnica 22. Jonsson NN, Mayer DG, Matschoss AL, Green PE, et al. (1998). Production effects of cattle tick (Boophilus microplus) infestation of high yielding dairy cows. Vet. Parasitol. 78: 65-77. Kelm SC, Detilleux JC, Freeman AE, Kehrli Jr ME, et al. (1997). Genetic association between parameters of inmate immunity and measures of mastitis in periparturient Holstein cattle. J. Dairy Sci. 80: 1767-1775. Kemp DH, Koudstaal D and Kerr JD (1971). Labelling larvae of the cattle-tick Boophilus microplus, with 32 P to follow their movements on the host. Parasitology 63: 323-330. Koppen W and Geiger R (1936). Handbuch der Klimatologie. Vol. 1. Part C. Verlag, Börntraeger, Berlin, Germany. Labarta V, Rodriguez M, Penichet M, Lleonart R, et al. (1996). Simulation of control strategies for the cattle tick Boophilus microplus employing vaccination with a recombinant Bm86 antigen preparation. Vet. Parasitol. 63: 131-160. Lie O and Solbu H (1981). A working model for the evaluation of different immunological traits as indicators of resistance to infection in dairy cattle. Acta Vet. Scand. 22: 238-245. Maillard JC, Renard C, Chardon P, Chantal I, et al. (1999). Characterization of 18 new BoLA-DRB3 alleles. Anim. Genet. 30: 200-203. Martinez ML, Silva MVGB, Machado MA, Nascimento CS, et al. (2004). Associação do gene candidato BOLA-DRB3.2 com resistência a ectoparasitos em bovinos. Proceedings of the 41 Mejdell CM, Lie O, Solbu H, Arnet EF, et al. (1994). Association of major histocompatibility complex antigens (BoLA-A) with AI bull progeny test results for mastitis, ketosis and fertility in Norwegian cattle. Anim. Genet. 25: 99-104. Moraes FR, Moraes JRE, Costa AJ, Rocha UF, et al. (1992). A comparative study of lesions caused by different parasitic stages of Boophilus microplus (Canestrini) in the skin of naturally infested taurine and zebuZebuine hosts. The correlation of tick resistance with mast cell counts in the host’s skin. Braz. J. Vet. Res. Anim. Sci. 29: 378-383. Park CA, Hines HC, Monke DR and Threlfall WT (1993). Association between the bovine major histocompatibility complex and chronic posterior spinal paresis - a form of ankylosing spondylitis - in Holstein bulls. Anim. Genet. 24: 53-58. Pegram RG, James AD, Oosterwijk GP, Killorn KJ, et al. (1991). Studies on the economics of ticks in Zambia. Exp. Appl. Acarol. 12: 9-26. Roth JA and Kaeberle ML (1981). Evaluation of bovine polymorphonuclear leukocyte function. Vet. Immunol. Immunopathol. 2: 157-174. SAS Institute (2002). SAS/STAT User’s Guide Version 8.0. 5th edn. SAS Institute, Inc., Cary, NC, USA. Sharif S, Mallard BA, Wilkie BN, Sargeant JM, et al. (1998). Associations of the bovine major histocompatibility complex DRB3 (BoLA-DRB3) alleles with occurrence of disease and milk somatic cell score in Canadian dairy cattle. Anim. Genet. 29: 185-193. Skow LC, Womack JE, Petresh JM and Miller WL (1988). Synteny mapping of the genes for 21 steroid hydroxylase, alpha A crystallin, and class I bovine leukocyte antigen in cattle. DNA 7: 143-151. Spooner RL, Leveziel H, Grosclaude F, Oliver RA, et al. (1978). Evidence for a possible major histocompatibility complex (BLA) in cattle. J. Immunogenet. 5: 325-346. Stear MJ, Dimmock CK, Newman MJ and Nicholas FW (1988). BoLA antigens are associated with increased frequency of persistent lymphocytosis in bovine leukaemia virus infected cattle and with increased incidence of antibodies to bovine leukaemia virus. Anim. Genet. 19: 151-158. Stear MJ, Newman MJ, Nicholas FW, Brown SC, et al. (1984). Tick resistance and the major histocompatibility system. Aust. J. Exp. Biol. Med. Sci. 621: 47-52. Stear MJ, Tierney TJ, Baldock FC, Brown SC, et al. (1989). Class I antigens of the bovine major histocompatibility system are weakly associated with variation in faecal worm egg counts in naturally infected cattle. Anim. Genet. 19: 115-121. Stear MJ, Hetzel DJ, Brown SC, Gershwin LJ, et al. (1990). The relationships among ecto- and endoparasite levels, class I antigens of the bovine major histocompatibility system, immunoglobulin E levels and weight gain. Vet. Parasitol. 34: 303-321. Szabó MPJ and Bechara GH (1990). Sequential histopathology at the Rhicephalus sanguineus tick feeding site on dogs and guinea pigs. Exp. Appl. Acarol. 23: 915-928. Teale AJ, Kemp SJ and Morrison WI (1991). The major histocompatibility complex and disease resistance in cattle. In: Breeding for disease resistance in farm animals (Owen JB and Axford RFE, eds.). CAB International, Wallingford, United Kingdom, 162-186. Teodoro RL and Madalena FE (2003). Dairy production and reproduction by crosses of Holstein, Jersey or Brown Swiss sires with Holstein-Friesian/Gir dams. Trop. Anim. Health Prod. 35: 105-115. Teodoro RL, Lemos AM and Madalena FE (1998). Effects of ticks Boophilus microplus infestations on milk yield of Bos taurus/Bos indicus crosses. Proceedings of the 6th World Congress on Genetics Applied to Livestock Production, Armidale, Australia, 137-180. van Eijk MJ, Stewart-Haynes JA and Lewin HA (1992). Extensive polymorphism of the BoLA-DRB3 gene distinguished by PCR-RFLP. Anim. Genet. 23: 483-496. Veríssimo CJ, Nicolau CVJ, Cardoso VL and Pinheiro MG (1996). Características do pelame e infestação por carrapatos em bovinos GirGyr e mestiços (Holandês x GirGyr). Proceedings of the 33 Veríssimo CJ, Silva RB, Oliveira AAD, Ribeiro WR, et al. (1997). Resistência e suscetibilidade de bovinos leiteiros mestiços ao carrapato Boophilus microplus. Bol. Ind. Anim. 54: 1-10. Warner CM, Meeker DL and Rothschild MF (1987). Genetic control of immune responsiveness: a review of its use as a tool for selection for disease resistance. J. Anim. Sci. 64: 394-406. Weigel KA, Freeman AE, Kehrli ME, Stear MJ, et al. (1990). Association of class I bovine lymphocyte antigen complex alleles with health and production traits in dairy cattle. J. Dairy Sci. 73: 2538-2546. Willadsen P (1980). Immunity to ticks. Adv. Parasitol. 18: 293-311. Xu A, van Eijk MJT, Park C and Lewin HA (1993). Polymorphism in BoLA-DRB3 exon 2 correlates with resistance to persistent lymphocytes caused by bovine leukaemia virus. J. Immunol. 151: 6977-6985. |
|