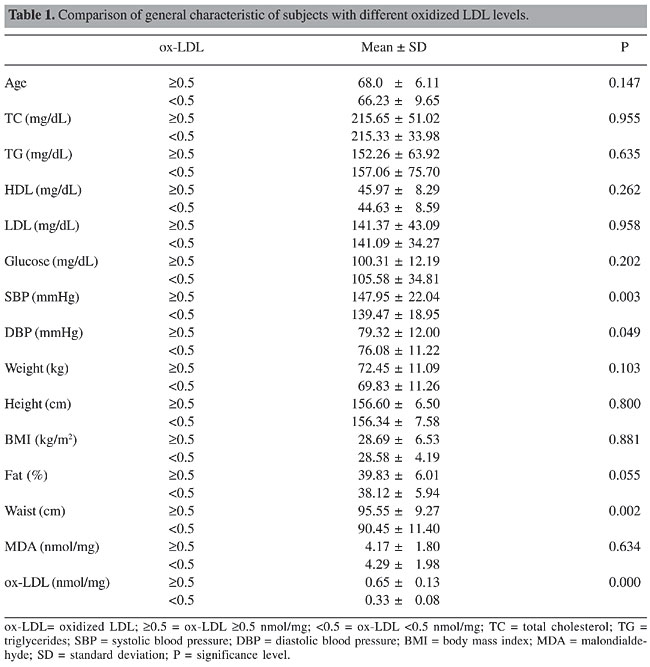
ABSTRACT. Oxidized LDL (ox-LDL) is involved in the initiation and progression of atherosclerosis. Many factors can affect the LDL oxidation such as oxidative stress. The present study tested whether ox-LDL levels would be associated with apolipoprotein E (APOE), manganese superoxide dismutase (MnSOD) Ala16Val polymorphisms, and classic cardiovascular risk factors. ox-LDL levels were measured by thiobarbituric acid-reactive substances and both polymorphisms were determined by polymerase chain reaction/restriction fragment length polymorphism in a sample of 252 subjects (70 men, 182 women, mean age, 54-85 years). Subjects with ox-LDL ³0.5 nmol/mg apoprotein were considered the high level group (HLG, N = 82) and subjects with ox-LDL <0.5 nmol/mg apoprotein were considered the expected level group (ELG, N = 170). Classic risk factors were also evaluated. The results showed that diabetes mellitus was more prevalent in HLG, whereas other cardiovascular risk factors were similar between groups. The APOE genotype frequencies did not differ between HLG and ELG subjects. However, AA genotype from MnSOD polymorphism was more frequent in ELG (c2 = 8.48; P = 0.014). AV and VV subjects from ELG present highest ox-LDL levels (OR = 3.61; CI95% = 1.42-9.17) than AA. Additional analysis did not find gene-gene interactions associated with ox-LDL levels. Multivariate analysis showed that diabetes and the MnSOD polymorphism were independent factors associated with higher ox-LDL levels in HLG. The results suggest that an important framework on modulation of the redox status influenced by genetic polymorphisms could affect the cardiovascular homeostasis. Key words: ox-LDL, Oxidative stress, APOE gene polymorphism, MnSOD gene polymorphism, Ala16Val polymorphism, Atherosclerosis, Oxidative damage INTRODUCTION The development of atherosclerosis is a complex process influenced by a network of risk factors, such as hypertension, hyperlipidemia, smoking, and genetic predisposition (Berliner et al., 1995). However, several investigations have suggested that atherosclerosis could be thought of as a chronic inflammatory disease with the basic abnormality lying in the redox-state of the vascular wall cells (Kunsch and Medford, 1999). The possible role of oxidative imbalance could contribute to atherogenesis from a differential modulation of the expression of several molecules such as cytokines and adhesion molecules (Kunsch and Medford, 1999). The increasing in the expression of these molecules probably promotes the invasion of inflammatory cells into the vessel wall, which is an early and important event in atherogenesis. Especially, the oxidation of low-density lipoproteins (ox-LDL) is considered to be a crucial step in the atherogenesis (Berliner et al.,1995). Whereas oxidative stress is involved in the pathogenesis of atherosclerosis, a variety of antioxidants have been used in clinical studies during the past few years for the prevention and treatment of atherosclerosis. Small clinical studies have found that both vitamins C and E might improve endothelial function in patients with risk factors for atherosclerosis such as diabetes mellitus, smoking, hypertension, or hypercholesterolemia. However, the initial and hopeful reports regarding the beneficial role of antioxidant vitamins against atherosclerosis, derived from purely observational studies, were followed by the negative results of almost all large randomized trials. Therefore, treatment with antioxidant vitamins C and E should not be recommended for the prevention or treatment of coronary atherosclerosis (Antoniades et al., 2003). This landscape suggested that the oxidative imbalance (more free radicals or more antioxidant compounds) could be responsible for the development of body dysfunctions involved in complex diseases as atherosclerosis. For this reason, several studies investigating the role of enzymes related to oxidative metabolism were performed in the last years, mainly involving the superoxide dismutases (SODs). SODs are enzymes catalyzing dismutation of reactive superoxide radicals to hydrogen peroxide and three SODs catalyze this reaction in mammals: manganese SOD (MnSOD) on the mitochondria, copper-zinc SOD on the cytosol and extracellular SOD in extracellular compartments (Zelko et al., 2002). Polymorphisms of neither the copper-zinc SOD gene nor the extracellular SOD gene were associated with the risk of macroangiopathy in patients with type 2 diabetes mellitus (Ukkola et al., 2001). Experimental studies with knockout animals strongly suggest that MnSOD plays a role of major importance in the oxidant resistance of vital organs (Li et al., 1995). Single amino acid polymorphism alanine (Ala) to valine (Val) at the 16th amino acid (16th amino acid from the beginning of the signal sequence or -9th amino acid from the first amino acid of the mature protein) of the signal sequence of the MnSOD (Ala16Val) has been suggested to change the secondary structure of the premature protein and therefore the mitochondrial targeting of the enzyme (Shimoda-Matsubayashi et al., 1996). Recent investigations have used in vitro import of chimeric proteins composed of either one of the MnSOD/MTS fused to the mouse dihydrofolate reductase protein, and the import of the two human MnSOD precursor variants into rat liver mitochondria. The results showed that the Ala-MnSOD precursor generated 30-40% more of the active matricial, processed MnSOD homotetramer, than the Val-MnSOD precursor. In the case, the Ala-MnSOD/MTS allows efficient MnSOD import into the mitochondrial matrix, while the Val-variant caused partial arrest of the precursor within the inner membrane and decreased formation of the active MnSOD homotetramer in the mitochondrial matrix (Sutton et al., 2003). So far, there are only a few studies linking MnSOD with heart diseases and atherosclerosis. Firstly, MnSOD locus has been linked to the atherogenic lipoprotein phenotype, i.e., the excess of small dense LDL in humans (Allayee et al., 1998). Overexpression of MnSOD has been shown to protect transgenic mice against myocardial ischemia (Chen et al., 1998) and in rabbits to reverse vascular dysfunction in carotid arteries without atherosclerotic changes, but not in vessels with atherosclerotic plaques (Zanetti et al., 2001). Overexpression of MnSOD inhibits in vitro oxidation of LDL by endothelial cells (Fang et al., 1998) and ox-LDL is able to induce the expression of MnSOD in macrophages (Kinscherf et al., 1997). The apoE-deficient mice lacking MnSOD had more severe atherosclerosis compared to the apoE-deficient mice (Ballinger et al., 2002). In addition, the signal sequence polymorphism of the MnSOD gene has been associated with non-familial dilated cardiomyopathy in Japanese subjects (Hiroi et al., 1999), but it has not been investigated earlier in human atherosclerosis. Based on the same theoretical approach Kakko et al. (2003) studied whether the signal sequence polymorphism of the MnSOD could be associated with the degree of carotid atherosclerosis. The author genotyped in a sample of 989 middle-aged hypertensive and control subjects the MnSOD polymorphism. Carotid atherosclerosis was quantified as intima-media thickness (IMT) by ultrasound. The results obtained suggested that signal sequence polymorphism could be a minor determinant of carotid IMT explaining 1.3% of the overall variation, the Val allele associated with the higher IMT. The results also suggested gender interaction. In women, a significant interaction with plasma levels of LDL cholesterol (LDL-c) was detected, since LDL-c levels were positively correlated with carotid IMT only in carriers of the Val allele, and the Val allele was associated with higher IMT only in subjects with high plasma levels of LDL-c. The authors concluded that the signal sequence polymorphism of the MnSOD gene is a minor determinant of carotid IMT, pointing out the importance of redox-balance in the atherogenesis. What other physiological and genetic variables could be influenced by the results obtained? To contribute to the elucidation of this question we performed a study between subjects with high ox-LDL and between expected range values comparing the classical cardiovascular risk, MnSOD and apolipoprotein E (APOE) polymorphism frequency distributions in a sample that live in the Southern Brazilian region (Rio Grande do Sul State). MATERIAL AND METHODS The study was structured considering the checklist for reporting and appraising of gene-disease associations proposed by Little et al. (2002). The population examined was composed of Caucasian volunteers. In this case, participants were recruited by random selection from the Health and Social Assistance Program of Gravataí, a city from the Porto Alegre Metropolitan area, Rio Grande do Sul State. All volunteers went to an outpatient visit to the research unit for laboratory tests, physical examination and interviews. Sample The sample consisted in 252 subjects (70 men and 182 women), ³50 years old, without cardiovascular disease history, grouped into two categories: A) high ox-LDL level group (HLG): formed by 82 subjects with ox-LDL levels ³0.5 nmol/mg apoprotein; B) expected ox-LDL level group (ELG): formed by 170 subjects with ox-LDL levels <0.5 nmol/mg apoprotein. Individuals using medicines to lower cholesterol and those with renal and hypothyroidism history were excluded. Variables analyzed Clinical variables The body mass index was calculated dividing weight (kg) by height squared (m2) (WHO, 1997). We used standard desk mercury sphygmomanometers (Wanross®) and stethoscopes (Littman®) to assess the blood pressure measurement (BP) (III Consenso Brasileiro de Hipertensão, 1998). BP was measured 30 min or more after the last caffeine intake or cigarette smoked. Three measures were taken at 5 min of the initial rest and subsequently at 2-min intervals, when an increased diastolic (DBP) or systolic BP (SBP) was recorded (mean DBP ³90 and/or mean SBP ³140 mmHg). Biochemical variables The following blood tests were performed: biochemical analysis (glucose, total cholesterol, HDL-c, LDL-c, and triglycerides, TG) (Tonks, 1972). Malondialdehyde values were measured using the biochemical approach described by Janero (1990). Blood samples were collected from Gravataí’s subjects after an overnight fasting (12 h or more); snacks and coffee were offered afterwards. Samples were sent to the Biochemistry and Molecular Genetics Laboratory of the Institute of Geriatrics and Gerontology at the Pontifícia Universidade Católica do Rio Grande do Sul, where they were analyzed. Total cholesterol, HDL-c, TG, and glucose were determined by enzymatic colorimetric methods using commercial kits: total cholesterol Cod-Ana Labtest®, HDL-c precipitant Labtest®, TG Gpo-Ana, Glucose PAP Labtest®, and LDL-c were calculated according to the Friedewald equation: (LDL-c) = (TG) - (HDL-c + TG/5). The plasma ox-LDL was estimated using thiobarbituric acid-reactive substance formation using the fluorimetric procedures of Yagi (1987). Cardiovascular risk factors a) Diabetes mellitus: individuals with glycemic levels above 126 mg/dL and those in use of medicines to lower glucose were considered diabetics; b) obesity: those with body mass index >30 were considered obese; c) sedentarism: those who performed physical activity less than tree times a week; d) tabagism: tobacco use was assessed by clinical history and the individuals were classified in smokers and non-smokers; e) dyslipidemia: subjects with total cholesterol, LDL-c or elevated TG were considered dyslipidemic as well as those who used drugs to lower cholesterol; f) hypertension: systolic blood pressure levels >140 mmHg or diastolic blood pressure levels >90 mmHg, or both, were considered hypertensive. Molecular variables Blood samples of a peripheral vein were withdrawn according to the venoclysis system with a disposable vacuum device (Vacutainer) and stored in tubes with 0.1% EDTA (final volume at the concentration of 1 mg/dL). After this, the material collected was maintained at 4°C until DNA extraction for up to 24 h. Genomic DNA was isolated from peripheral blood leukocytes using a GFX Genomic Blood DNA Purification (Amersham Biosciences Inc., Co.) kit. The following polymorphism genotypings were performed (Lahiri and Nurnberger Jr., 1991): 1. MnSOD genotyping. PCR-RFLP methods were done at a total volume of 50 µL containing 5.0 µL 10X buffer, 1.0 µL 25 mM MgCl2, 1.25 µL 10 mM dNTP, 0.5 µL Taq polymerase (Gibco Inc., Co.), 1.0 µL 40 pmol each primer, 3.0 µL Genomic DNA (0.25 µg), 34.5 µL ddH2O. The amplification primers (Gibco Inc., Co.) for a 110-bp fragment of the human SOD gene were 5’-ACCAGCAGGCAGCTGGCGCCGG-3’, (sense-strand) and 5’-GCGTTGATGTGAGGTTCCAG-3’ (antisense-strand) with thermocycler parameters comprised of an initial cycle of 95°C for 5 min followed by 35 cycles at 95°C for 1 min, 61°C for 1 min. The final cycle was followed by an extension period of 2 min at 72°C. PCR product (10 µL) was digested with HhaeIII (15 U; at 37°C, for 6 h, Gibco. Inc, Co.). Digested products (23 and 85 bp) were visualized on a 6% agarose gel (Amersham Biosciences Inc., Co.) stained with ethidium bromide. A mutation was introduced by a primer mismatch to create a restriction cut site for HhaeIII in the -9 codon, though the following genotypes were observed: -9Ala/Ala (23 and 85 bp); -9Ala/Val (23.85 and 110 bp), and -9Val/Val (110 bp) (Taufer, 2003). 2. APOE genotyping. PCR-RFLP were performed according to the method reported by James et al. (1990) using the primers apo EF 5'- TAA GCT TGG CAC GGC TGT CCA AGG A-3' (25-mer) and apo ER 5'- ACA GAA TTC GCC CCG GCC TGG TAC AC-3' (26-mer). The reaction’s mixture (total of 50 µL) was prepared with 10 pmol each primer, 100 µM dNTPs, 1 U Taq polymerase (CenBiot-RS), buffer containing 1.5 mM MgCl2, 1% DMSO, and 2 µL DNA of the sample. The amplification process consisted of an initial denaturation at 95ºC for 5 min, followed by 30 cycles of denaturation at 95ºC for 1 min, coiling at 61ºC for 1 min, extension at 70ºC for 1 min, and a final extension at 72ºC for 10 min. The efficacy of the PCR was visualized through electrophoresis on 2% agarose gel with ethidium bromide, when a band of 240 bp was observed under ultraviolet light. Genotyping was performed by digesting the PCR product (20 µL) with 5 U of the restriction enzyme HhaI (Gibco). The fragment sizes from polymorphic HhaI sites after cleavage were as follows: E2/E2: 91 and 83 bp; E3/E3: 91, 48, and 35 bp; E4/E4: 72, 48, and 35 bp; E3/E2: 91, 83, and 48 bp; E4/E2: 91, 83, 72, and 48 bp; E4/E3: 91, 72, and 48 bp. Statistical analysis The results are reported as means ± standard deviation or percentages. All the analyses were carried out using the statistical package for social studies SPSS version 11.0 (Inc., Chicago, IL). The differences between HLG and ELG were evaluated by the Student t-test for quantitative variables and by the chi-square test for categorical variables. Allele frequencies were estimated by gene-counting method. Chi-square (c2) analysis was used to estimate the Hardy-Weinberg equilibrium and to compare genotypic and allelic frequencies among the ethnic groups. The alpha value considered was P = 0.05. All P values were two-tailed. A P value of <0.05 was considered to be statistically significant. To test intervenient factors we performed a multivariate analysis using the Forward Wald logistic regression. The Research Ethics Committee of the Pontifícia Universidade Católica do Rio Grande do Sul approved the study protocol (No. 537/02) and informed consent was obtained from all individuals whose information was collected prospectively. RESULTS The baseline characteristics of HLG and ELG subjects are shown in Table 1.
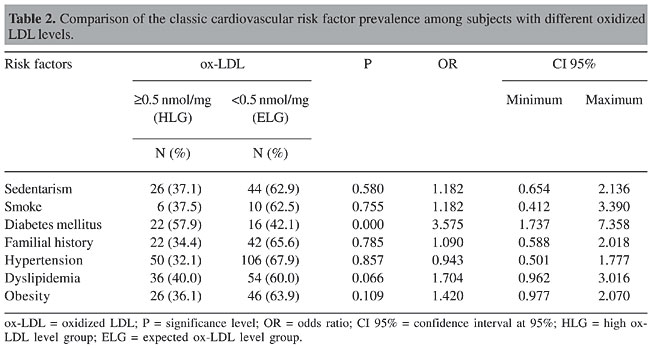
The frequency of gender in the HLG and ELG was similar (c2 = 0.05, P = 0.943). The analysis showed higher SBP, DBP, fat body composition, and abdominal fat values in the HLG. The prevalence of classic cardiovascular risk was compared between groups. The results obtained are presented in Table 2. The diabetes mellitus was prevalent in HLG, whereas the prevalence of the other cardiovascular risk factors was similar between groups shown in Table 2. Logistic regression showed that association among SBP, DBP, fat body composition, and abdominal fat values was related with diabetes II.
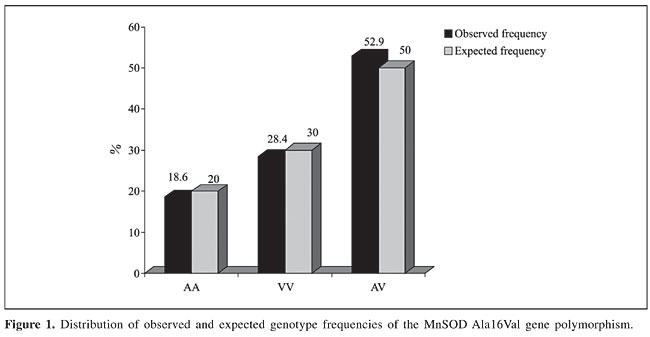
The MnSOD and APOE genotypes were in Hardy-Weinberg equilibrium. The MnSOD genotype frequencies were calculated and are presented in Figure 1. The MnSOD allelic frequency was A = 0.451 and V = 0.549.
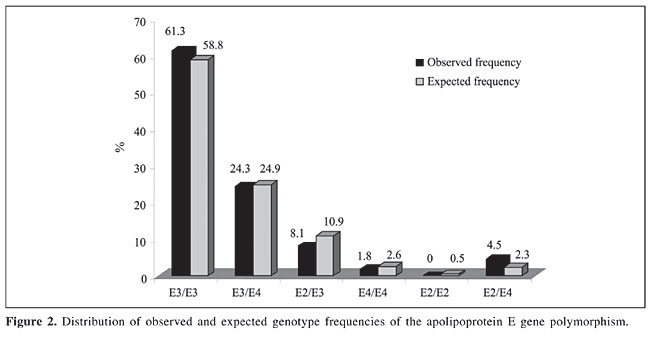
The APOE genotype frequencies were calculated and are presented in Figure 2. The frequencies were in Hardy-Weinberg equilibrium. The APOE allelic frequency was E3 = 0.767, E4 = 0.162, E2 = 0.071.
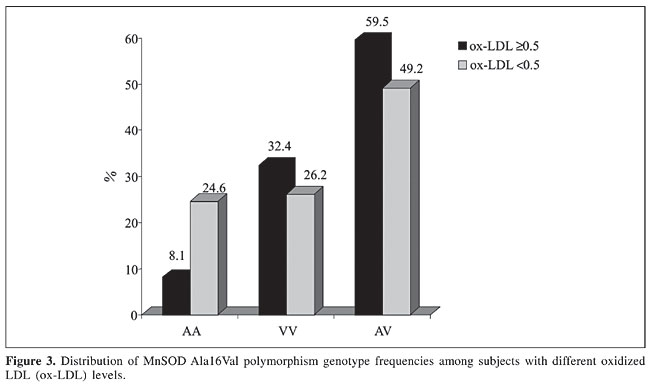
The comparison of MnSOD polymorphism frequencies between HLG and ELG showed an increase in the AA genotypes in the ELG (c2 = 8.48, P = 0.014) (Figure 3). The ox-LDL levels in AV + VV subjects together was high (³0.5 nmol/mg apoprotein), with odds ratio of 3.61 (CI 95% = 1.42-9.17).
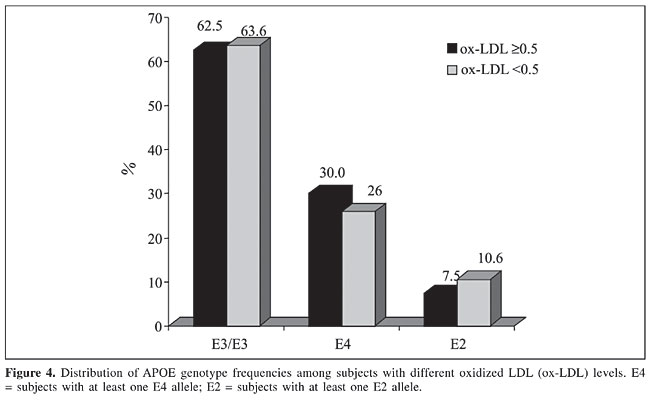
The APOE genotypes did not differ between HLG and ELG and their frequencies are shown in Figure 4.
The multivariate analysis showed that diabetes and Ala16Val polymorphism were independent factors associated with higher ox-LDL levels presented in the HLG. DISCUSSION The results described here show significant association among higher ox-LDL levels, biological traits and MnSOD polymorphism interaction related to diabetes II. Higher SBP, DBP, body fat (%), and waist circumference levels were observed in subjects with higher ox-LDL. However, these associations were diabetes II dependent. On the other hand, none association between the APOE polymorphism and the variable studied was observed. The ox-LDL and biological trait associations described here were observed in previous studies. Holvoet (2004) identified several metabolic syndrome components (high TG, low HDL-c, glucose intolerance, and diabetes) that independently of LDL-c, predicted high levels of ox-LDL. However, whereas some studies suggest that LDL oxidizability may be increased in people with type 2 diabetes (Dimitriadis et al., 1995; Schwenke et al., 2003) and that lipid peroxidation in such individuals is particularly high when glycemic control is poor (Nourooz-Zadeh et al., 1997; Schwenke et al., 2003), other investigations did not described this association (Oranje et al., 1998; Julier et al., 1999). These apparently contradictory results could be explained by gene-gene or gene-environmental associations related to ox-LDL and diabetes II. Here we observed possible interaction between higher ox-LDL levels and Ala16Val polymorphism with diabetes II. It is widely believed that oxidative stress plays an important role in the pathogenesis of type II diabetes. Therefore, the interaction between ox-LDL and MnSOD polymorphism on diabetes observed here could have a physiological explanation. The many studies on oxidative stress, antioxidant treatment, and diabetic complications have shown that oxidative stress is increasing and may accelerate the development of complications through the metabolism of excessive glucose and free fatty acids in diabetic and insulin-resistant states. In excess, reactive oxygen species (ROS) and their bioproducts that are capable of causing oxidative damage may be cytotoxic and increase the risk of atherosclerosis. Recent insights into the etiology and pathogenesis of atherosclerosis suggest that this disease may be seen as an inflammatory disease linked to an abnormality in oxidation-mediated signals in the vasculature. Generally the pathophysiological process of atherosclerosis is accelerated in diabetic subjects mainly when there are factors such as hyperglycemia, insulin resistance, abnormal lipid profile, oxidative modification of lipoproteins, increased blood pressure, altered rate of fibrinolysis, etc. (Scott and King, 2004). The possible association between AV + VV genotypes, ox-LDL and diabetes II could be explained by the decrease in the functional MnSOD enzyme into mitochondria caused by mutation and change in protein conformation that is observed in V allele carriers. However, we do not know if this mutation could affect the oxidative balance in the cytosol and extracellular medium. This is an open question that needs to be tested. On the other hand, association between MnSOD levels and diabetes II has been described and indirectly corroborate the results found in this study. Carmeli et al. (2004) investigated the function of two antioxidant scavenger enzymes, SOD and glutathione peroxidase (GSH-Px), in erythrocytes in a population of healthy aging adult women compared to a similar population with type II diabetes. A significant increase in SOD activity was correlated to aging in erythrocytes of the healthy control subjects. However, this correlation was not found in subjects with type II diabetes. The result indicates a possible imbalance in the antioxidant system in erythrocytes of aging adult women, which is even more pronounced in cases of type II diabetes. Another study performed by Manea et al. (2004) investigated the role of diabetic conditions such as high glucose, AGE-Lysine, and angiotensin II in the modulation of antioxidant enzyme activities, GSH level and ROS production in pericytes. The activity of antioxidant enzymes: SOD, catalase, GSH-Px, and total GSH were evaluated. The results indicated that diabetic conditions induce in pericytes: i) an increase of ROS and SOD activity, ii) a decrease in GSH-Px activity and GSH level, and iii) major perturbation of the intracellular calcium homeostasis. In this context, genetic decrease in SOD2 could increase oxidative stress and increase ox-LDL mainly in diabetic subjects. Additionally, it is important to comment that several investigations have described the modulation of ox-LDL by oxidative balance (Berliner et al., 1995; Kinscherf et al., 1997; Fang et al., 1998; Ballinger et al., 2002). Endothelial cells produce superoxide anions (-O2) and ox-LDL in vitro; however, the role of (-O2) in endothelial cell-induced LDL oxidation is unclear. Fang et al. (1998) performed an in vitro study incubating the human LDL with bovine aortic endothelial cells for 18 h, which resulted in a 4-fold increase in LDL oxidation compared to cell-free incubation malondialdehyde/mg LDL protein. The possible association with ox-LDL could be through modulation of molecules related to inflammatory processes such as cytokines and adhesion molecules (Wen et al., 2002). Probably, the physiological association between diabetes, ox-LDL levels and atherosclerosis occurs through inflammatory modulation. Taking into account these studies we suggest that genetic MnSOD level function observed in V allele carriers and diabetes could increase the LDL oxidation as observed here. We did not find any association between APOE and ox-LDL levels. Despite the APOE polymorphism being extensively studied and associated with the atherosclerosis process, there are few studies that analyzed the association between APOE polymorphism and ox-LDL levels. Among these studies we can cite the investigation performed by Chen et al. (2003) that examined the association between 3 polymorphisms in lectin-like ox-LDL receptor-1 gene (LOX1 or OLR1) with coronary artery disease in the Women’s Ischemia Syndrome Evaluation (WISE) study population. The results described by the authors showed that APOE and LOX1 genotypes were independently associated with the risk of disease and that there was no interaction between the two genes. The cited study indirectly corroborates to our results but do not show association between APOE polymorphism and ox-LDL levels. Moreover, the APOE could influence the atherosclerosis process for other metabolic pathways. The results described here suggest a possible interaction between MnSOD, ox-LDL and diabetes II. Additional investigations looking for possible other gene-gene or gene-environmental interactions on ox-LDL modulation (e.g., nutrition) could be important to understand the role of these variables on physiological and pathological processes such as atherogenesis. ACKNOWLEDGMENTS We thank all GENESIS Program researchers who help us to collect clinical data. Research supported by grants and fellowships from the Coordenação de Aperfeiçoamento de Pessoal (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), and the Municipal Authority of Gravataí-RS. The authors would like to thank Ivo Emílio Jung for the translation of the present study. REFERENCES Allayee H, Aouizerat BE, Cantor RM, Dallinga-Thie GM et al. (1998). Families with familial combined hyperlipidemia and families enriched for coronary artery disease share genetic determinants for the atherogenic lipoprotein phenotype. Am. J. Hum. Genet. 63: 577-585. Antoniades C, Tousoulis D, Tentolouris C, Toutouzas P et al. (2003). Oxidative stress, antioxidant vitamins, and atherosclerosis. From basic research to clinical practice. Herz 28: 628-638. Ballinger SW, Patterson C, Knight-Lozano CA, Burow DL et al. (2002). Mitochondrial integrity and function in atherogenesis. Circulation 106: 544-549. Berliner JA, Navab M, Fogelman AM, Frank JS et al. (1995). Atherosclerosis: Basic mechanisms. Oxidation, inflammation, and genetics. Circulation 91: 2488-2496. Carmeli E, Coleman R and Berner YN (2004). Activities of antioxidant scavenger enzymes (superoxide dismutase and glutathione peroxidase) in erythrocytes in adult women with and without type II diabetes. Exp. Diabesity Res. 5: 171-175. Chen Q, Reis SE, Kammerer C, Craig WY, et al. (2003). Genetic variation in lectin-like oxidized low-density lipoprotein receptor 1 (LOX1) gene and the risk of coronary artery disease. Circulation 107: 3146-3151. Chen Z, Siu B, Ho YS, Vincent R et al. (1998). Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J. Mol. Cell. Cardiol. 30: 2281-2289. III Consenso Brasileiro de Hipertensão Arterial (1998). BHTA, Campos do Jordão, SP, Brazil, p. 38. Dimitriadis E, Griffin M, Owens D, Johnson A et al. (1995). Oxidation of low-density lipoprotein in NIDDM: its relationship to fatty acid composition. Diabetologia 38: 1300-1306. Fang X, Weintraub NL, Rios CD, Chappell DA et al. (1998). Overexpression of human superoxide dismutase inhibits oxidation of low-density lipoprotein by endothelial cells. Circ. Res. 82: 1289-1297. Hiroi S, Harada H, Nishi H, Satoh M et al. (1999). Polymorphisms in the SOD2 and HLA-DRB1 genes are associated with nonfamilial idiopathic dilated cardiomyopathy in Japanese. Biochem. Biophys. Res. Commun. 261: 332-339. Holvoet P (2004). Oxidized LDL and coronary heart disease. Acta Cardiol. 59: 479-484. James E, Hixson JE and Vernier DT (1990). Restriction isotyping of human apoliprotein E by gene amplification and cleavage with HhaI. J. Lipid Res. 31: 545-548. Janero DR (1990). Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 9: 515-540. Julier K, MacKness MI, Dean JD and Durrington PN (1999). Susceptibility of low- and high-density lipoproteins from diabetic subjects to in vitro oxidative modification. Diabet. Med. 16: 415-423. Kakko S, Paivansalo M, Koistinen P, Kesaniemi YA et al. (2003). The signal sequence polymorphism of the MnSOD gene is associated with the degree of carotid atherosclerosis. Atherosclerosis 168: 147-152. Kinscherf R, Deigner HP, Usinger C, Pill J et al. (1997). Induction of mitochondrial manganese superoxide dismutase in macrophages by oxidized LDL: its relevance in atherosclerosis of humans and heritable hyperlipidemic rabbits. FASEB J. 11: 1317-1328. Kunsch C and Medford RM (1999). Oxidative stress as a regulator of gene expression in the vasculature. Circ. Res. 85: 753-766. Lahiri DK and Nurnberger Jr JI (1991). A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 19: 5444. Li Y, Huang TT, Carlson EJ, Melov S et al. (1995). Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 11: 376-381. Little J, Bradley L, Bray MS, Clyne M et al. (2002). Reporting, appraising, and integrating data on genotype prevalence and gene-disease associations. Am. J. Epidemiol. 156: 300-310. Manea A, Constantinescu E, Popov D and Raicu M (2004). Changes in oxidative balance in rat pericytes exposed to diabetic conditions. J. Cell. Mol. Med. 8: 117-126. Nourooz-Zadeh J, Rahimi A, Tajaddini-Sarmadi J, Tritschler H et al. (1997). Relationships between plasma measures of oxidative stress and metabolic control in NIDDM. Diabetologia 40: 647-653. Oranje WA, Rondas-Colbers GJ, Swennen GN and Wolffenbuttel BH (1998). Lipid peroxidation in type 2 diabetes: relationship with macrovascular disease? Neth. J. Med. 53: 61-68. Schwenke DC, D’Agostino Jr RB, Goff Jr DC, Karter AJ et al. (2003). Differences in LDL oxidizability by glycemic status: the insulin resistance atherosclerosis study. Diabetes Care 26: 1449-1455. Scott JA and King GL (2004). Oxidative stress and antioxidant treatment in diabetes. Ann. N.Y. Acad. Sci. 1031: 204-213. Shimoda-Matsubayashi S, Matsumine H, Kobayashi T, Nakagawa-Hattori Y et al. (1996). Structural dimorphism in the mitochondrial targeting sequence in the human manganese superoxide dismutase gene. A predictive evidence for conformational change to influence mitochondrial transport and a study of allelic association in Parkinson’s disease. Biochem. Biophys. Res. Commun. 226: 561-565. Sutton A, Khoury H, Prip-Buus C, Cepanec C et al. (2003). The Ala16Val genetic dimorphism modulates the import of human manganese superoxide dismutase into rat liver mitochondria. Pharmacogenetics 13: 145-157. Taufer M (2003). Associação do polimorfismo do gene da superóxido dismutase dependente de manganês (SOD2) com doenças crônico-degenerativas associadas ao envelhecimento. Doctoral thesis, Programa de Pós-Graduação em Gerontologia Biomédica da Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, RS, Brazil. Tonks DB (1972). Quality control in clinical laboratories. Warner-Chilcott Laboratories, Diagnostic Reagent Division, Scarborough, Canadá. Ukkola O, Erkkila PH, Savolainen MJ and Kesaniemi YA (2001). Lack of association between polymorphisms of catalase, copper-zinc superoxide dismutase (SOD), extracellular SOD and endothelial nitric oxide synthase genes and macroangiopathy in patients with type 2 diabetes mellitus. J. Intern. Med. 249: 451-459. Wen Y, Skidmore JC, Porter-Turner MM, Rea CA et al. (2002). Relationship of glycation, antioxidant status and oxidative stress to vascular endothelial damage in diabetes. Diabetes Obes. Metab. 4: 305-308. World Health Organization (1997). Obesity: preventing and managing the global epidemic (report of a WHO consultation on obesity). World Health Organization, Geneva, Switzerland. Yagi K (1987). Lipid peroxides and human diseases. Chem. Phys. Lipids 45: 337-351. Zanetti M, Sato J, Jost CJ, Gloviczki P et al. (2001). Gene transfer of manganese superoxide dismutase reverses vascular dysfunction in the absence but not in the presence of atherosclerotic plaque. Hum. Gene Ther. 12: 1407-1416. Zelko NI, Mariani JT and Folz JR (2002). Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution and expression. Free Radic. Biol. Med. 33: 337-349. |
|