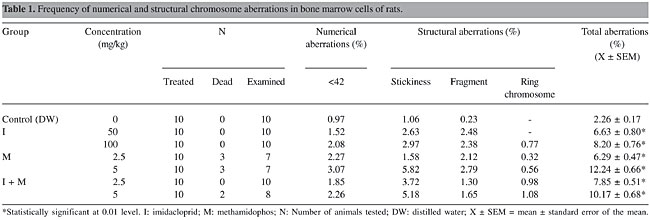
ABSTRACT. We examined the cytogenetic and genotoxic effects of the neonicotinoid insecticide imidacloprid and the organophosphate insecticide methamidophos, when administered alone or in combination. These insecticides were tested with the bone marrow chromosome aberration assay and micronucleus test in rats and by the bacterial mutation assay (Salmonella/microsome mutagenicity assay). Wistar albino rats were orally fed daily with laboratory chow treated with various concentrations of insecticides, 50 and 100 mg/kg imidacloprid, 2.5 and 5 mg/kg methamidophos, and 2.5 and 5 mg/kg imidacloprid plus methamidophos, respectively, for 90 days. Numerical and structural chromosomal aberrations were evaluated. Significant differences were detected between all the insecticide-administered groups versus the control group and between the two concentrations of the pesticide-treated groups. Both concentrations of the insecticides induced a dose-related increase in the micronucleus frequency (P < 0.05). Dose-related increases in the number of revertants were observed with the two Salmonella strains (TA98 and TA100). All tested doses of the insecticides demonstrated mutagenic activity in the presence of S9 mix. These results lead us to the conclusion that the synergistic effect of methamidophos and imidacloprid causes an increase in potential damage to non-target organisms. Key words: Imidacloprid, Methamidophos, Chromosome aberration, Micronucleus, Rat, Salmonella microsome assay INTRODUCTION Pesticides, in addition to their intended effects, are sometimes found to affect non-target organisms, including humans (Chantelli-Forti et al., 1993; Chaudhuri et al., 1999). Genotoxicity of pesticides for non-target organisms and their influence on ecosystems are of worldwide concern (Pimentel et al., 1998). Because of the potential environmental impact connected with the introduction and heavy use of pesticides, it is necessary that the genotoxic potential of these agents be studied. Previous studies have demonstrated that some pesticides have mutagenic and clastogenic activities in several biological test systems (Shirasu et al., 1976; Degraeve and Moutschen, 1984; Ashby et al., 1993; Jayashree et al., 1994; Sierra-Tores et al., 1998; Siroki et al., 2001; Celik, 2003). However, additional well-conducted in vitro and in vivo genotoxic studies are necessary to assess the possible health risks associated with the extensive use of pesticides. Methamidophos is a highly active, systemic, residual organophosphate insecticide. It is a potent acetylcholinesterase inhibitor, and it is effective against both chewing and sucking insects (Hussain, 1987). This compound is highly toxic to mammals, birds, and bees (Kidd and James, 1991; Meister, 1995). Imidacloprid is an agonist at the nicotinic acetylcholine receptor, and as such it is highly effective against many sucking insects (Worthing, 1994; Elbert et al., 1998). This chemical works by interfering with the transmission of stimuli in the insect nervous system. It causes a blockage in a type of neuronal pathway that is more abundant in insects than in warm-blooded animals. This blockage leads to the accumulation of acetylcholine, resulting in the insect’s paralysis, and eventually death (Kidd and James, 1991). Although pest control studies have recently been performed on synergistically activated pesticides, scientific studies of these synergisms are very limited. As the use of insecticides has become increasingly widespread throughout the world, additional studies are needed to evaluate the potential toxic risk of insecticides for non-target organisms. We examined the genotoxicity of two of these insecticides, when administered alone, or in combination, for their synergistic effect. MATERIAL AND METHODS Chemicals Methamidophos (Tamaron®), imidacloprid (Confidor®), and a methamidophos plus imidacloprid combination (Taifun®) were purchased from Bayer Chemical Corporation in Turkey. All other chemicals used were of analytical grade. Study design The genotoxicity assays were performed at the ARGEFAR Laboratory (Turkey). The 90-day subchronic study was conducted in accordance with OECD (1998) guideline 408. Cytogenetic analysis was performed with a bone-marrow chromosome-aberration assay, based on the recommendations of Adler (1984), with slight modifications. The slight modifications were a decrease in colchicine concentration and colchicine application time. The frequency of micronucleated erythrocytes in femoral bone marrow was evaluated according to the procedures of Schmid (1976) and Feng (2000), with slight modifications in centrifugation time. The bacterial mutation assay was performed in accordance with OECD (1997) guideline 471 and standard procedures previously described by Maron and Ames (1983). Animals and subchronic 90-day oral toxicity study The protocols were approved by the Animal Ethics Committee of the Faculty of Medicine of Ege University. Ten-week-old male (N = 70) Wistar albino rats (100 g) were obtained from the Ege University, Faculty of Medicine, Center for Animal Breeding. All animals were acclimatized for 10 days before starting the experimental procedure. The rats were assigned randomly to either a control or a test group and were housed in individual cages (19 x 19 x 12 cm) with solid plastic sides and stainless-steel grid tops and floors in a room with temperature of approximately 21 ± 1°C, 45-75% humidity and a 12-h light/dark cycle. Ten rats of the control group were orally fed daily with a normal diet composed of standard laboratory chow combined with corn oil (10 g/day per rat), while the same number of animals of the test group were orally fed daily with laboratory chow (10 g/day per rat) containing different concentrations of each insecticide dissolved in corn oil, 50 and 100 mg/kg imidacloprid, 2.5 and 5 mg/kg methamidophos, and a combination of 2.5 and 5 mg/kg imidacloprid plus methamidophos. Animals were treated with these insecticides for 15 weeks according to OECD (1998) guideline 408. Tap water was also available ad libitum. Food and water were changed daily (Francis et al., 1990). Concentrations of insecticides in the diet were calculated based on 10 and 20% of their LD50 values and on body weight data. Bone marrow chromosome aberration assay At the end of the 90-day oral toxicity study, the animals were sacrificed by cervical dislocation, and the genotoxic effect was evaluated by the bone marrow chromosome aberration assay (Adler, 1984). About 2 h before sacrifice, colchicine (4 mg/kg) (Sigma Chem. Corp., USA) was injected intraperitoneally to produce mitotic arrest. Both femora were dissected out and cleaned of any adhering muscle. The femoral bone marrow was aspirated into 8 mL 0.075 M KCl (37°C) and the cells were flushed out with a Pasteur pipette. The cell suspension was incubated at 37°C for 20 min. The cells were centrifuged at 2000 g for 10 min, and the supernatant was removed. After centrifugation, the cells were resuspended in 7 mL fixative (3:1 methanol:glacial acetic acid). Tubes with the cell suspension were kept at +4°C overnight. Centrifugation and fixation (in the cold) were repeated four times at intervals of 20 min. Slides were prepared by the air-drying method. The slides were stained the following day for 10 min in 8.5 mL 5% buffered Giemsa solution, pH 6.8. Twenty slides were prepared for each animal. One hundred cells were examined to detect the chromosomal aberrations in each slide. Chromosome numbers, specific types of chromosomes and chromatid-type aberrations were evaluated. The data were analyzed by the Fisher exact test to determine if there was significant increase in aberrant cells in the insecticide-treated groups compared with the control. Statistical analysis was performed by SPSS 10.0. Micronucleus test The frequency of micronucleated erythrocytes was evaluated based on a technique developed by Schmid (1976) and Feng (2000). At the end of the 90-day oral toxicity study, the animals were sacrificed by cervical dislocation. The femoral bone marrow was flushed out using 1 mL of fetal calf serum and centrifuged at 2000 g for 10 min. The supernatant was discarded. Smears were prepared for each animal, fixed in methanol and stained with 5% Giemsa in Sörensen buffer. Smears were screened at a magnification of 1000X, using a light microscope. The erythrocytes with one or more micronuclei were counted in at least 2000 erythrocytes per animal. Micronucleus frequency (MN º/oo) was calculated as follows: MN º/oo = (number of cells containing micronucleus/total number of cells counted) x 1000. Statistical differences between control and concentration groups were determined by the Dunnet test. Bacterial mutation assay The assay was performed in two histidine-requiring strains of Salmonella typhimurium, tester strains TA98 and TA100, according to OECD (1997) guideline 471 and Maron and Ames (1983). Two separate experiments were performed, using triplicate plates, in the presence and absence of metabolic activation by an Aroclor 1254-induced (500 mg/kg body weight) Wistar albino rat liver post-mitochondrial fraction (S9). The post-mitochondrial fraction was used at a concentration of 10% v/v in the S9 mix. The S9 mix was freshly prepared for each experiment according to the method of Maron and Ames (1983). Negative controls and positive controls were tested in all strains in both experiments. The positive controls were 5 µg/plate benzo[a]pyrene, 5 µg/plate 2-nitrofluorene and 10 µg/plate sodium azide. Fresh cultures of tester strains were grown to approximately 109 cell/mL in 5 mL Oxoid nutrient broth No. 2. The cultures were incubated for 10-12 h at 37°C in a gyratory incubator in order to insure adequate aeration. The strains were periodically raised from a single colony to check the genetic markers. Four concentrations of each insecticide (25, 50, 75, 100 µL per plate for imidacloprid, 2.5, 5, 7.5, and 10 µL per plate for methamidophos, and 2.5, 5, 7.5, and 10 µL per plate of the imidacloprid plus methamidophos combination) were tested on TA98 and TA100. Insecticide concentrations were plated in triplicate with 0.1 mL of overnight bacterial cultures per plate. The solutions were prepared in the absence (0.5 mL/plate phosphate buffer 1 M, pH = 7.4) and presence of S9 mix (0.5 mL/plate). The mixture containing chemicals and bacteria with or without S9 was vortexed and pre-incubated at 37°C for 30 min. It was then plated in 2 mL of top agar on glucose-supplemented minimal agar. After 48 h of incubation at 37°C, revertant colonies (his +) were counted. RESULTS The frequency of chromosomal aberrations increased with increasing concentrations of the insecticides (Table 1). Chromosomal aberrations were determined as numerical chromosomal aberrations, which consisted of a decrease in chromosome number (<42), and structural chromosomal aberrations, which consisted of sticky chromosomes, fragments and ring chromosomes. Significant differences were detected between all the insecticide-administered groups versus the control group and between the two concentrations of the insecticide-administered groups (P < 0.01).
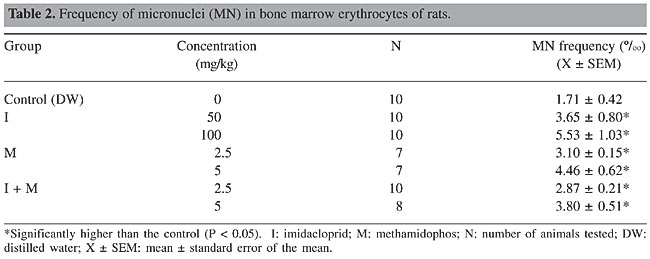
Imidacloprid, methamidophos and the imidacloprid plus methamidophos combination induced a dose-related increase in micronucleus frequency (Table 2). Both doses of these insecticides significantly increased the micronucleus frequency when compared to the control (P < 0.05).
Although spontaneous levels of mutations differed among the various Salmonella strains, dose-related increases in the number of revertants were observed for all the strains (Table 3). Five- to six-fold increases in strain TA98 and around a two-fold increase in strain TA100 in the number of revertants compared to the spontaneous rate were observed. Various concentrations were evaluated when the assay was carried out in Salmonella strains, both in the presence and the absence of metabolic activation, depending on the results obtained in the test. Addition of a metabolic activation system in the form of rat liver homogenate increased the number of revertants by 50-60% in these two strains. An increase was observed with the TA100 strain, but only with metabolic activation, and this level was always found to be higher than twice the spontaneous number of revertants observed in the control. When the chemical concentrations and resulting mutation frequencies produced by imidacloprid, methamidophos and the combined preparation were compared with those produced by controls, all tested doses of the insecticides demonstrated mutagenic activity in the presence of S9 mix (Table 3).
DISCUSSION Short-term genotoxic assays for the detection of potential human carcinogens have been used by many investigators and have been validated in international collaborative programs (ICPEMC, 1988). The in vitro Salmonella/microsome assay with rat-liver microsomal fraction S9 is one of the most frequently used tests for assessing the mutagenic potential of both pure compounds and complex mixtures. In addition, the United States, Environmental Protection Agency (US EPA) recommends that a positive in vitro response be confirmed by an in vivo assay, since some chemicals producing positive responses in the Salmonella/microsome assay have shown negative results when in vivo evaluations are made (Dearfield et al., 1991; Auletta et al., 1993). Short-term test series, including combinations of the Salmonella/microsome assay together with the chromosome aberration test in mammalian cells, were found to significantly improve the sensitivity for detection of potential human carcinogens. The chromosome aberration assay is used extensively in population monitoring, since it is recognized as a reliable biomarker for documentation of genotoxic effects due to exposure (Sierra-Tores et al., 1998). The induction of chromosomal aberrations and micronucleated erythrocytes due to exposure to imidacloprid and methamidophos indicates that they have a potential for clastogenicity. Chromosomal aberrations significantly increased (P < 0.01) with both concentrations of the three insecticides. An increased rate of these aberrations is considered indirect evidence of external pesticide treatment. In our study, these data also indicate the cytotoxic potential of these insecticides. This was also found in other studies on the cytotoxic potential of insecticides (El-Nahas et al., 1988; Flessel et al., 1993; Jayashree et al., 1994). Mutations were induced in the TA100 strain, which responds to base-pair substitutions, and in strain TA98, which is sensitive to frame-shift mutations. The methamidophos apparently was sufficiently persistent in the plate to increase the number of revertants/plate, a phenomenon that was also reported by Ivanovic et al. (1985), Sierra-Tores et al. (1998) and Aiub et al. (2002), using other organophosphate compounds, and by Ruiz and Marzin (1997) and Feng et al. (2004), using other insecticides, and by Wagner et al. (2003), using synergistically acting insecticides. We showed that the insecticides were able to induce G-C base pair mutations (Maron and Ames, 1983), causing a frame shift reversion of the histidine-dependent tester strains (TA98 and TA100) to the wild type (his +). Consequently, these responses also indicate that the insecticides by themselves, and with metabolic activation by S9, are indirectly acting mutagens. These results are in agreement with other studies on the positive genotoxicity and weakly mutagenic potential of methamidophos (Extoxnet, 1993). However, imidacloprid, methamidophos and the combined preparation were found to be indirect-acting mutagens in the Salmonella/microsome assay, and dose-dependent chromosome aberration induction was demonstrated in the rat bone-marrow assay. However, this could be attributed to a synergistic toxic effect from the multiple chemical exposure (imidacloprid and methamidophos) and the prolonged exposure to the two doses of the insecticides. One mechanism of the organophosphate-mediated mutagenic synergy could be a reaction that generates products with higher mutagenic activity. If there were a simple molecular relationship between methamidophos and imidacloprid, a maximum synergistic effect would be reached with both agents. In our opinion, there is no simple molecular relationship between methamidophos and imidacloprid. In conclusion, methamidophos and imidacloprid were found to be genotoxic and mutagenic in the three tests used. These results lead us to the conclusion that the synergistic effect of methamidophos and imidacloprid towards the target organisms may cause an unwanted increase in the potential damage to the non-target organisms. Synergistic responses are rarely incorporated in risk assessment models. However, such responses are extremely important in establishing the real toxicological characteristics of agents that impact upon the environment and public health. Potential genotoxic hazard in humans after exposure to low concentrations of insecticides for a long period of time should also be investigated. ACKNOWLEDGMENTS The present study was done at the Ege University Center for Drug R&D and Pharmacokinetic Applications and was supported by the Ege University Research Foundation (Project number: 96/FEN/031). The authors thank Prof. Dr. Is1k Tuglular, M.D., Assoc. Prof. Necip Tosun and Dr. Ak1n Denizci for their help. REFERENCES Adler ID (1984). Cytogenetic tests in mammals. In: Mutagenicity testing, a practical approach (Venitt S and Parry JM, eds.). IRL Press, Oxford, England, UK, pp. 275-306. Aiub CAF, Coelho ECA, Sodre E, Pinto LFR et al. (2002). Genotoxic evaluation of the organophosphorus pesticide temephos. Genet. Mol. Res. 1: 159-166. Ashby J, Anwar W, Au WW, Massoud A et al. (1993). Genetic toxicology in developing countries: comments and recommendations. Environ. Health Perspect. 101 (Suppl 3): 335-338. Auletta AE, Dearfield KL and Cimino MC (1993). Mutagenicity test schemes and guidelines: U.S. EPA Office of Pollution Prevention and Toxics and Office of Pesticide Programs. Environ. Mol. Mutagen. 21: 38-45. Celik A, Mazmanci B, Camlica Y, Askin A et al. (2003). Cytogenetic effects of lambda-cyhalothrin on Wistar rat bone marrow. Mutat. Res. 539: 91-97. Chantelli-Forti G, Paolini M and Hrelia P (1993). Multiple end point procedure to evaluate risk from pesticides. Environ. Health Perspect. 101: 15-20. Chaudhuri K, Selvaraj S and Pal AK (1999). Studies on the genotoxicity of endosulfan in bacterial systems. Mutat. Res. 439: 63-67. Dearfield MC, Auletta AE, Cimino MC and Moore MM (1991). Considerations in the US Environmental Protection Agency’ s testing approach for mutagenicity. Mutat. Res. 258: 259-283. Degraeve N and Moutschen J (1984). Genetic and cytogenetic effects induced in the mouse by an organophosphorus insecticide: malathion. Environ. Res. 34: 170-174. Elbert A, Nauen R and Leicht W (1998). Imidacloprid, a novel chloronicotinyl insecticide: biological activity and agricultural importance. In: Insecticides with novel modes of action (Ishaaya I and Degheele D, eds.). Springer, Berlin, Germany, pp. 50-73. El-Nahas SM, De-Hondt HA and Ramadan AI (1988). In vivo evaluation of the genotoxic potential of curacron in somatic cells of mice. Environ. Mol. Mutagen. 11: 515-522. Extoxnet (1993). Extension Toxicology Network Pesticide Information Profiles. Accessed http://extoxnet.orst.edu/pips/methamid.htm. Feng S, Kong Z, Wang X, Zhao L et al. (2004). Acute toxicity and genotoxicity of two novel pesticides on amphibian, Rana N. Hallowell. Chemosphere 56: 457-463. Feng SL, Wu D, Zhang GD and Kong ZM (2000). Effects of two new pesticides on the micronucleus formation in mice and tadpoles. Environ. Sci. Res. 19: 137-138. Flessel P, Quintana PJ and Hooper K (1993). Genetic toxicity of malathion: a review. Environ. Mol. Mutagen. 22: 7-17. Francis AJ, Anderson D, Evans JG, Jenkinson PC et al. (1990). Tumours and malformations in the adult offspring of cyclophosphamide - treated and control male rats - preliminary communication. Mutat. Res. 229: 239-246. Hussain MA (1987). Anticholinesterase properties of methamidophos and acephate in insects and mammals. Bull. Environ. Contam. Toxicol. 38: 131-138. ICPEMC (1988). International Commission for Protection Against Environmental Mutagens and Carcinogens. Publication No. 16. Testing for mutagens and carcinogens; the role of short-term genotoxicity assays. Mutat. Res. 205: 3-12. Ivanovic V, Rapic V and Boskovic B (1985). Pinacolyl methylphosphonochloridate: in vitro covalent binding to DNA and mutagenicity in the Ames test. Mutat. Res. 142: 9-12. Jayashree IV, Vijayalaxmi KK and Abdul RM (1994). The genotoxicity of Hinosan, an organophosphorus pesticide in the in vivo mouse. Mutat. Res. 322: 77-85. Kidd H and James DR (1991). The agrochemicals handbook. 3rd edn. Royal Society of Chemistry Information Services, Cambridge, UK (as updated). Maron DM and Ames BN (1983). Revised methods for the Salmonella mutagenicity test. Mutat. Res. 113: 173-215. Meister RT (1995). Farm chemicals handbook 95. Meister Publishing Company, Willoughby, OH, USA. OECD (1997). Guideline for testing of chemicals. Bacterial reverse mutation test. Guideline 471 (Updated guideline adopted July 21, 1997). OECD (1998). Repeated dose 90-day oral toxicity study in rodents. Guideline 408 (Updated September 21, 1998). Pimentel D, Greiner A and Bashore T (1998). Economic and environmental costs of pesticide use. In: Environmental toxicology: current developments (Rose J, ed.). Gordon and Breach Science Publishers, London, UK, pp. 121-187. Ruiz MJ and Marzin D (1997). Genotoxicity of six pesticides by Salmonella mutagenicity test and SOS chromotest. Mutat. Res. 390: 245-255. Schmid W (1976). The micronucleus test for cytogenetic analysis. In: Chemical mutagens: Principles and methods for their detection (Hollaender A, ed.). Vol. 4. Plenum Press, New York, NY, USA, pp. 31-53. Shirasu Y, Moriya M, Kato K, Furuhashi A et al. (1976). Mutagenicity screening of pesticides in the microbial system. Mutat. Res. 40: 19-30. Sierra-Tores CH, Cajas-Salazar N, Hoyos LS, Zuleta M et al. (1998). In vitro and in vivo genotoxic activity of miral, an organophosphorus insecticide used in Colombia. Mutat. Res. 415: 59-67. Siroki O, Undeger U, Institoris L, Nehez M et al. (2001). A study on geno- and immunotoxicological effects of subacute propoxur and pirimicarb exposure in rats. Ecotoxicol. Environ. Saf. 50: 76-81. Wagner ED, Marengo MS and Plewa MJ (2003). Modulation of the mutagenicity of heterocyclic amines by organophosphate insecticides and their metabolites. Mutat. Res. 536: 103-115. Worthing CR (1994). The pesticide manual. A world compendium. 10th edn. British Crop Protection Council, Croydon, UK. |
|