ABSTRACT. Diploid males have long been considered a curiosity contradictory to the haplo-diploid mode of sex determination in the Hymenoptera. In Apis mellifera, ‘false’ diploid male larvae are eliminated by worker cannibalism immediately after hatching. A ‘cannibalism substance’ produced by diploid drone larvae to induce worker-assisted suicide has been hypothesized, but it has never been detected. Diploid drones are only removed some hours after hatching. Older larvae are evidently not regarded as ‘false males’ and instead are regularly nursed by the brood-attending worker bees. As the pheromonal cues presumably are located on the surface of newly hatched bee larvae, we extracted the cuticular secretions and analyzed their chemical composition by gas chromatograph-mass spectrometry (GC-MS) analyses. Larvae were sexed and then reared in vitro for up to three days. The GC-MS pattern that was obtained, with alkanes as the major compounds, was compared between diploid and haploid drone larvae. We also examined some physical parameters of adult drones. There was no difference between diploid and haploid males in their weight at the day of emergence. The diploid adult drones had fewer wing hooks and smaller testes. The sperm DNA content was 0.30 and 0.15 pg per nucleus, giving an exact 2:1 ratio for the gametocytes of diploid and haploid drones, respectively. Vitellogenin was found in the hemolymph of both types of imaginal drones at 5 to 6 days, with a significantly lower titer in the diploids. Key words: Apis mellifera, Diploid and haploid drones, Vitellogenin, Larval cuticular hydrocarbons, Adult size, Sperm DNA content, Wing hooks INTRODUCTION The haplo-diploid mode of sex determination is widespread in the animal kingdom, being found in approximately 20% of all extant species (Bull, 1983), and it is often combined with heterozygosity in the diploid sex (Crozier, 1971). This is particular true for the Hymenoptera (Cook, 1993). In diplo-diploid insects, such as Drosophila and other dipterans, different sex-determining systems have evolved, usually including sex chromosomes (Schutt and Nöthiger, 2000). The karyotype of male and female honey bees was first described in studies of gametogenesis and embryogenesis (Petrunkewitsch, 1901; Nachtsheim, 1913). The pioneer discovery that drones are derived from unfertilized eggs (Dzierzon, 1845) was followed by the definition of parthenogenesis based on studies of butterflies and bees (Siebold, 1856), which was confirmed by Paulcke (1899). Diploid males from fertilized eggs were found only much later in the Hymenoptera (Whiting and Whiting, 1925). Biparental males were produced by inbreeding the parasitic wasp Habrobracon juglandis (now Bracon hebetor), which led to the hypothesis of ‘complementary sex determination’, with multiple alleles at a single sex locus (Whiting, 1943). According to this genetic model, Hymenopteran females are always heterozygous, and the males are homozygous, or hemizygous for the sex locus in the case of haploids. Therefore, diploid drones result from match-mating of queens and drones with identical sex alleles. The number of existing multiple alleles was determined for the first time as about 19 in a population of 90 colonies of Africanized bees in Brazil (Adams et al., 1977). Long before the tools of molecular genetics became available, possible mechanisms of sex determination in bees were proposed (Kerr and Nielson, 1967) and the genetic load at sex-limited loci was discussed (Kerr, 1969). The validity of the five main original hypotheses concerning the genetics of sex determination in the Hymenoptera was recently examined again by Kerr (1997). Diploid honey bee drones are effectively inviable, thus resulting in ‘shot-brood combs’ (Mackensen 1951, 1955). In closely inbred colonies, obtained by artificial insemination, about half of the brood in worker-sized comb cells disappears, but in adult bees indications of drone diploidy have only been found in some gynandromorphs, which had mosaics of zygotic male tissue in their compound eyes (Rothenbuhler, 1957; Drescher and Rothenbuhler, 1964). That the ‘false males’ really hatch (Woyke, 1962) from fertilized eggs (Woyke, 1965) but are eliminated within a few hours by the brood-attending worker bees, which ate the diploid drone larvae, was shown by (Woyke, 1963a,b,c,d). His explanation assumed a suicidal signal released by the diploid males, which he named ‘cannibalism substance’ (Woyke, 1967). However, such a compound was never found (Woyke, 1967; Dietz, 1975; Dietz and Lovins, 1975; Bienefeld et al., 1994, 2000; Santomauro et al., 2004). Instead, a different quantitative pattern in the cuticular hydrocarbons of newly hatched diploid drone larvae in comparison to those of workers and haploid drones was shown to elicit removal behavior by the worker bees (Santomauro et al., 2004). As already described by Woyke (1969 a,b), diploid drone larvae survive if they are reared for some time outside the colony, and then replaced in a colony one or two days later; after this period they will not be cannibalized any more. The reason for this remarkable change in the worker reaction towards diploid drones remained unknown. Using a recently improved method of sexing live first-instar bee larvae (Santomauro and Engels, 2002), based on the epiproct characters already used for this purpose by Kerr and Nielson (1967), we now can work with pure samples of newly hatched diploid drone larvae. Only a few studies have been carried out on properties of adult diploid drones, as they are difficult to rear. Morphometrically, they more closely resemble normal haploid drones than they do diploid workers and queens (Woyke, 1977, 1978a; Chaud-Netto, 1975), including body weight at emergence (Woyke, 1978b). Their testes were found to be small (Woyke, 1973). In haploid and diploid drones, as in all Hymenoptera, male gametogenesis is ameiotic, and consequently the sperm contains clonal copies of the drone’s haploid DNA complement (Woyke and Skowronek, 1974). In all species of honey bees, the male haploid karyotype comprises 16 chromosomes and the female diploid set 32 chromosomes (Fahrenhorst, 1977). Twice the DNA content of haploid drones was found in the spermatozoa produced by diploid drones (Woyke, 1975). The yolk precursor protein vitellogenin is not strictly female specific in the honey bee (Trenczek and Engels, 1986). Adult haploid drones also produce vitellogenin, mainly during the two weeks after emergence (Trenczek et al., 1989), but the hemolymph titer is much lower in the males than in females, and especially when compared to queens (Engels et al., 1990). The honey bee vitellogenin gene was recently sequenced (Piulachs et al., 2003). Information on vitellogenin occurrence was not available for diploid drones. The shape (Snodgrass, 1956) and the number of wing hooks vary among individual bees; the mean numbers differ sex specifically (Drescher, 1972; Gonçalves, 1972, 1976). Nothing was known on hook numbers in diploid drones. During the past 40 years, after the spectacular discovery of diploid drones in the honey bee, various peculiar characters have been analyzed in these ‘false males’; however, comparisons with haploid drones are often lacking. MATERIAL AND METHODS Bees Colonies of Carniolan Apis mellifera in the apiary of the University of Tübingen were used for all the experiments and analyses. Virgin queens were instrumentally inseminated (Ruttner, 1975; Kühnert, 1991; Skowronek et al., 1995) with the semen of about six son or brother drones. These inbred colonies produced approximately 50 or 25% diploid drones, and consequently they had to be reinforced by adding brood combs or young worker bees from time to time. The hive entrances were protected with a queen excluder to prevent mating flights. Morphometry Adult diploid drones were obtained by in vitro rearing of male diploid larvae for three days; these were fed with a semi-artificial diet composed of royal jelly with sugars, vitamins and a fungicide (Herrmann and Engels, 2005). After this interval, the diploid drone larvae were grafted into drone brood cells, from which haploid L3 or L4 drone larvae had been removed, and the combs were re-introduced into normal non-inbred colonies. There, the diploid drones were reared until a day or two before emergence, and then placed in an incubator at 34°C. We also collected drones reared in worker-sized brood combs in queenless colonies and from queenright colonies; the latter had emerged from drone brood combs. Emerged adult drones were weighed within 12 h (yet unfed) to the nearest 0.1 mg and then kept in Kirchhainer nuclei (styrofoam boxes for small colonies of up to 2,000 bees; Tozetto et al., 1997) or queenless colonies, provided with plenty of young worker bees and a queen excluder screen at the entrance. They were collected from these hives five to six days later, weighed again and then dissected for wing and testis morphometry, sperm DNA measurements and vitellogenin electrophoresis. Wing hooks were counted in 14 diploid drones, in 15 haploid brother drones, in 20 diploid sister workers, and in addition in 31 haploid drones and 51 diploid workers sampled during the season of reproduction from several unrelated colonies headed by naturally mated queens. The individual mean number of hooks per wing was used to calculate the average, because sometimes the right and the left hind wing differed in the number of hooks. Larval sampling and extraction Brood combs with numerous eggs were placed in an incubator at 35°C and 80% humidity and were inspected for hatching larvae. Young L1 instars were collected from worker-sized brood cells every 2 h and separated into workers and their diploid drone brothers (Santomauro and Engels, 2002). In addition, newly hatched normal haploid male larvae were collected from drone brood combs. The larvae were subsequently reared in vitro in 96-well microtiter trays (Figure 1) and fed on a semi-artificial diet (Figure 2) consisting of lyophilized royal jelly, redissolved and enriched with sugars, vitamins and a fungicide (Herrmann and Engels, 2005). Every 12 h the larvae were transferred onto fresh food. In order to facilitate this mass rearing, an apparatus for automatic feeding of the larvae was designed (Herrmann and Engels, 2005). Samples of five of these larvae, reared outside the colony and therefore uncontaminated because they had no contact with adult bees, were removed, briefly rinsed in lukewarm water to remove adhering larval food and extracted in 0.5 ml pentane (Merck, uvasol grade) for 60 s in order to collect compounds from the cuticular surface. The extracts were stored at -20°C until analysis.
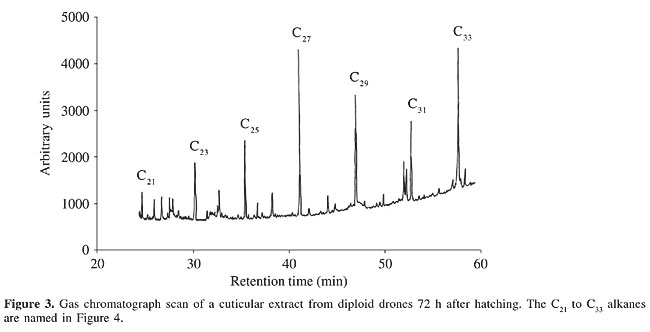
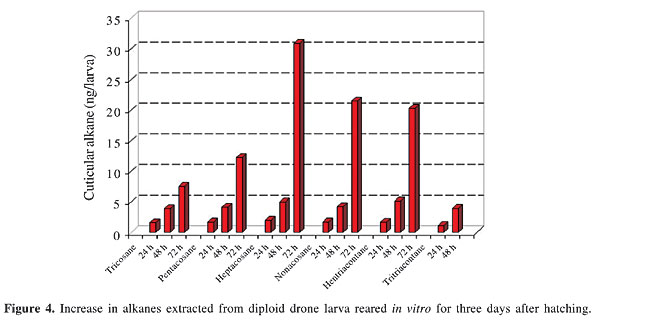
Combined gas chromatography and mass spectrometry analysis of larval cuticular compounds The larval cuticular extracts were analyzed by gas chromatography and mass spectrometry (GC-MS). Hewlett Packard equipment (HP 6890 GC and HP 5973 MS) was used, fitted with a 10 m DB-5 MS column, with 0.25 mm internal diameter, covered with a polydimethyl siloxane phase. The carrier gas was helium (10 ml/min) and injections were made splitless. The temperature was initially kept at 60°C for 2 min, then increased by 10°C/min to 120°C, followed by 5°C/min to 290°C, and finally kept for 20 min at 290°C. Linear compounds were identified by retention times in comparison with synthetic standards of equivalent chain length based on data in the NIST MS library, and methyl-branched compounds were determined by common fragmentation patterns. Several aliquots of each pentane wash of five live larvae were subjected to GC-MS analysis, with reproducible results. Vitellogenin content of the hemolymph Hemolymph samples were collected into capillaries and stored at -20°C, until analysis. In drones, a wing was cut off and the leaking blood was sampled. In worker bees, an incision was made into the anterior abdominal tergites. This was done to prevent hemolymph contamination from the intestinal contents. Electrophoretic separation of the proteins was done on cellogel strips (Chemetron, Milano), using a Tris-EDTA borate buffer adjusted to pH 9.2, for 6 h, at 10°C, with 140 V and 1.2 mA/strip, resulting in anodal migration of all hemolymph proteins. Amido black staining was done in 9:1 methanol/acetic acid. The vitellogenin band was identified by immunoreaction with a specific polyclonal antiserum (data not shown) prepared by rabbit immunization with purified honey bee vitellogenin. The vitellogenin content of the hemolymph was determined by two-dimensional quantitative immuno electrophoresis in 1% agarose gel with Tris buffer, pH 8.6, and Coomassie blue staining (Laurell, 1965), using the polyclonal anti-vitellogenin serum. The standards for calibration were prepared with bovine serum albumin. Sperm DNA measurements Testes of 5- to 6-day-old adult drones were dissected and fixed in 3:1 ethanol/acetic acid for 30 min, followed by 2 min of maceration in 1:1 acetic acid/water. Squash preparations were rinsed and hydrolyzed for 40 min in 5 N HCl, rinsed again and Feulgen stained in Schiff’s reagent for 30 min in the dark at 5°C. After 5 min washout in acid Na-metabisulfide solution the preparations were dehydrated, embedded in eukitt (EMS, Hartfield, PA, USA) mounting medium and protected with a cover slide. After drying, the measurements were made with a universal micro-photometer (Zeiss UMSP II), equipped with a rapid meanderscan unit, under 560-nm monochromatic light, using an ultrafluar 100 objective 1.25 and glycerol intermedium. The recorded arbitrary units were calculated in pg/sperm nucleus by co-measured chicken blood preparations containing 2.34 pg per diploid erythrocyte nucleus (Mirsky and Ris, 1951). About 20 spermatozoon nuclei were measured per drone. Statistical analysis Data were analyzed by the Student t-test (two sided for unrelated samples; the significance threshold (a) was 5%). RESULTS Larvae Cuticular compounds A large number of peaks were seen in the gaschromatic scans of the cuticular extracts; among which we concentrated on the alkanes as the main hydrocarbon compounds (Figure 3). The general pattern obtained for larval extracts of the diploid drones corresponded to those of haploid drones and workers (data not shown). No additional substances were detected exclusively in the cuticular secretions of the diploid males at any time after larval hatching. However, we found large differences in the amounts of the major alkanes (Figure 3) in the diploid drone extracts in the samples taken after 24 h, 48 h and 72 h of in vitro rearing of the larvae (Figure 4).
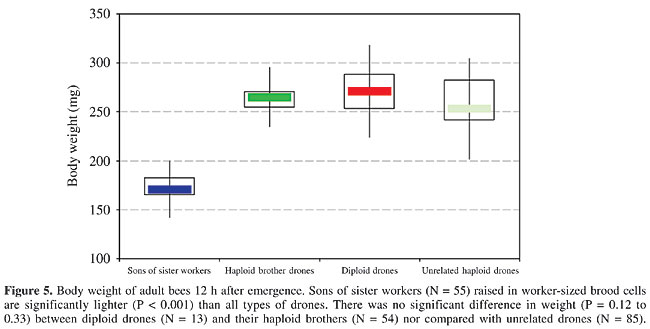
The 23 to 33 C hydrocarbons were present only in moderate quantities on the diploid drone larvae 24 h after hatching. After 48 h the amounts of these alkanes had increased considerably. The quantitative change in pattern continued with age, and after 72 h of in vitro rearing (Figure 3), some of the compounds were present in 10-fold amounts when compared with the 24-h sample (Figure 4). This increase corresponds with the growth of the larvae and the resulting much larger body surface. Adults Weight at emergence The diploid drones and their haploid brothers had the same body weight range (Figure 5). The worker-son drones from the queenless colonies (reared in worker-size cells) were significantly lighter than the drones reared in drone comb.
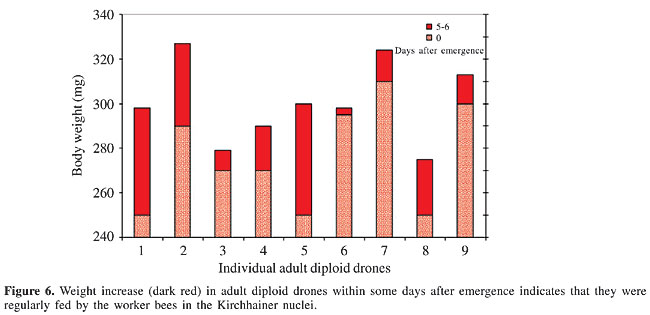
Weight of 5- to 6-day-old drones When the drones were kept in a queenless colony, their body weight increased (Figure 6). The diploid males must have obtained food from the workers.
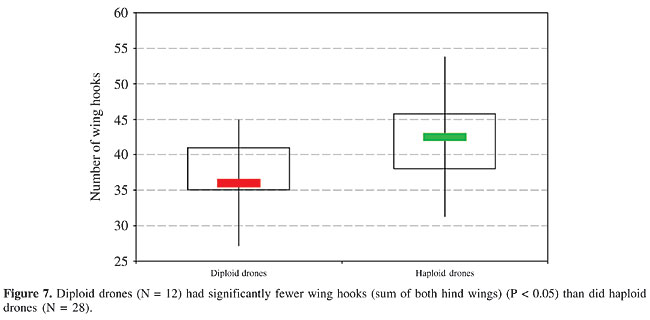
Number of wing hooks There was a difference in the number of hooks on the right and the left hind wing in most of the diploid drones, ranging between 1 and 5 hooks; this was occasionally also seen in normal drones and in workers. Consequently, we summed both wings for the comparison of the diploid and haploid drones (Figure 7). The diploid males had significantly fewer wing hooks than the haploids (P < 0.05); however, there was some variability within the two types of males. Sister workers had on average 21.4 hooks per hind wing, with less variability, which was closer to the haploid males with 22.5 than to the diploid drones with 19.3.
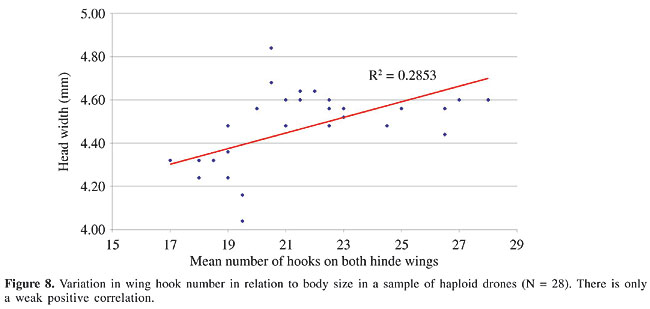
We plotted the distribution of the mean number of hooks per wing in relation to the individual size for a sample of haploid drones from another population, using head width as a constant reference, independent of the body weight (Figure 8). These drones had a head width of 4 to 5 mm. Most of the smaller males had fewer wing hooks, numbering from 17 to 20. In the larger individuals up to 28 hooks per hind wing were counted. There was considerable variation in this sample, with an overall correlation between head size and wing hook number of R2 = 0.285 (Figure 8).
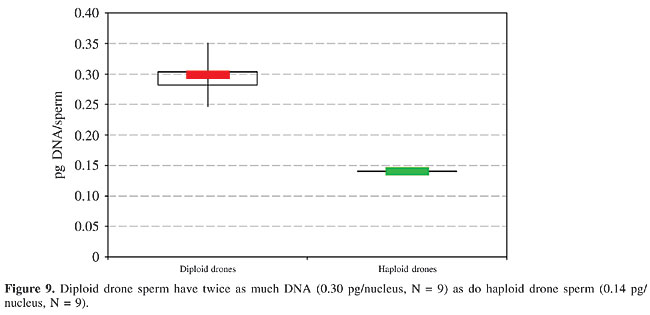
Testis size There was a tremendous difference in the size of the gonads between diploid and haploid drones. In one-day-old imaginal bees, the testes of big haploid drones with a volume of about 10 mm3 were about 50 times larger than the smallest testes of some diploid males with just 0.2 to 0.3 mm3. On average in the newly emerged diploid drones the size of the testes was 5-10% of the normal size. These data are based on volume estimates as calculated from dissection of not less than 10 live individuals. Haploid and diploid drones 5 to 14 days old had somewhat larger gonads. The mucus glands of the diploid drones were at least about half the size of those of the haploid drones, measuring about 5 mm2. DNA per spermatozoon The DNA content per sperm nucleus was determined as 0.30 pg in the preparations of the diploid testes and 0.14 pg in those of the haploid testes (Figure 9). This gives a 2:1 ratio, as expected for 2n and n chromosomal complements, respectively. There was only a slight variation in the values obtained for the individual diploid males, which is apparently due to differences in the Feulgen staining and the extinction values, and does not indicate varying DNA contents.
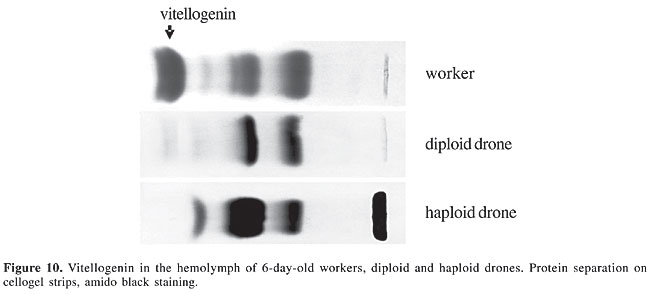
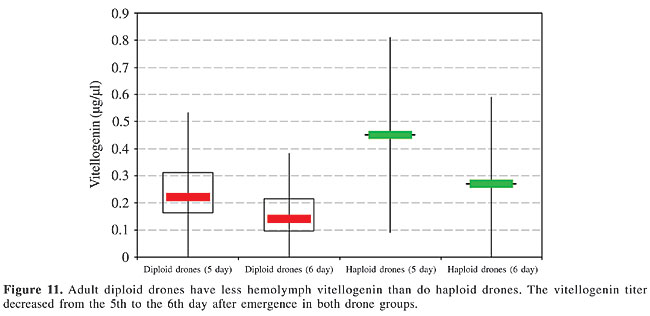
Vitellogenin As indicated by the delicate, faint bands, vitellogenin was found in the hemolymph of five-day-old diploid and haploid males in only trace amounts (Figure 10). Based on the measurement of the absolute hemolymph titer, the vitellogenin concentration was significantly lower in the diploid drones than in the haploids, with a slight decrease already apparent within one more day of adult age, similar in both types of males (Figure 11).
DISCUSSION Molecular aspects of Hymenopteran complementary sex determination Progress in molecular techniques has recently allowed sequencing of the complete genome of the honey bee (Tyler, 2004), now making possible a comparison of the loci involved in sex determination and differentiation within various invertebrates represented in gene banks such as Drosophila and Caenorhabditis. In Apis mellifera, not only the locus csd (Beye et al., 2003; see also Butcher et al., 2000), but also the vitellogenin gene (Piulachs et al., 2003), have already been deciphered. When the nucleotide sequence of sex alleles of a number of geographic honey bee populations, including the Africanized strain in Brazil (Hasselmann, 2001), was compared, a similarity with the tra gene involved in sex determination (by RNA splicing) in Drosophila (Heinrichs et al., 1998) became apparent. Perhaps we will soon understand how the pathway of male versus female development in bees is initiated by the csd transcript (Hasselmann and Beye, 2004). A new insight into the role of csd has permitted the suppression of transcription at this locus, by application of dsRNA, which results in male gonadal development in diploid heterozygotes, which are genetically destined to become females (Beye et al., 2002, 2003). This means that the sex option under ‘loss-of-function’ conditions at the csd locus is male, which makes it highly probable that the homozygotic allele constellation also inhibits the transcription of the gene product that is essential for primary steps of the female pathway in bee development. Only much later, juvenile hormone is involved in caste-specific expression of genes controlling ovary morphogenesis in female pupae to determine imaginal and functional queen or worker status, also by altering the rate of ecdysteroid biosynthesis (Hepperle and Hartfelder, 2001). Because diploid drones produce diploid sperm, which would result in aneuploid lethal progeny, their fitness is zero, confirmed by their complete elimination in the honey bee colony. Occurrence of diploid drones in bees Diploid males have been found in many hymenopterans. Within the Apini (Michener, 2000), diploid drones are also known in the following taxa, comprising solitary species such as Andrena ferox (Leys, 1994), as well as social bees, including Apis cerana (Woyke, 1979, 1980; Hoshiba et al., 1981), Bombus atratus (Garófalo, 1973; Garófalo and Kerr, 1975), Bombus terrestris (Duchateau et al., 1994), Melipona quadrifasciata (Camargo, 1979), Mormoniella vitripennis (Saul et al., 1965), and Tetragonula quadrangular (Tarelho, 1973). In most of the non-Apis species, males can copulate repeatedly. The lack of fitness of diploid drones can therefore be compensated by the reproductive activity of normal haploid males. However, in the social species, and in particular in the honey bee, the diploid drones are scheduled to become workers, and their elimination diminishes the strength of the colony. Recently, Paxton et al. (2003) detected male maternity in the stingless bee Scaptotrigona postica by microsatellite analysis; in two colonies 50% of the male pupae were diploid, and these colonies were weak. Due to the mass provisioning and immediately post-oviposition sealing of the brood cells in meliponids, there is no chance for recognition and removal of the ‘false’ diploid male larvae. Of course, the chance of diploid drone occurrence depends principally on the queen’s mating frequency, which is, though with a low degree of polyandry, also sometimes multiple in stingless bees (Paxton et al., 1999). Comparison of diploid and haploid drones in the honey bee By means of our recently developed technique of sexing live first-instar bee larvae (Santomauro and Engels, 2002), we are now able to collect pure samples of large numbers of diploid drones, providing sufficient material for chemical analysis. The removal of newly hatched diploid larvae by nursing workers was recently bioassayed in our lab (Santomauro et al., 2004), indicating that the reaction is released by an odd quantitative pattern in the cuticular hydrocarbons. This pattern is already altered after 24 h, in larvae reared completely in vitro, without any contamination by adult bee contact (Herrmann and Engels, 2005). We have shown here that within 72 h after hatching the diploid drone-specific label increasingly diminishes, changing into the secretional image of normal haploid drones. In diploid, as well as in haploid honey bee larvae, the quantity of cuticular hydrocarbons increases with larval growth and is correlated with enlargement of the body surface. In first-instar unfed honey bee larvae, the amounts of cuticular hydrocarbons can be ranked as In our sample of freshly emerged adult males of Carniolan strain, there was no weight difference between diploid and haploid drones, when they were reared in drone combs. Therefore, such diploid males could not be distinguished by weighing, and likewise not by any morphometric measurements. In contrast to our results, Woyke (1978b) reported that diploid drones, obtained from Africanized and Italian colonies in Brazil, were about 25% heavier than the haploids. A good approach to recognize adult diploid drones is to count the wing hooks. Drescher (1971, 1972) and Gonçalves (1972, 1976) used this purely quantitative morphological character in experiments on disruptive selection. In our sample, the diploid drones had significantly fewer wing hooks than their haploid brothers. If the data on wing hook numbers of related workers are included, they rank in between diploid and haploid drones: 2n Woyke (1973, 1974) found the testes of diploid drones of various bee races to generally be 10 times smaller than those of haploid drones, both derived from the same queen. In newly emerged drones of our much smaller sample of diploid and haploid brother males we found greater size differences, with a lot of variation. However, the mucus gland volume data of Woyke (1973) were similar to what we calculated. Our measurements of the sperm DNA confirmed the diploid state of spermatozoa produced by diploid drones. Woyke (1975) also found twice as much DNA in the spermatozoa of diploid drones compared to those of haploids, without calculating the absolute amounts. Our data for the diploid spermatozoa (0.30 pg/nucleus) are similar to the 0.35 pg measured for diploid somatic cells (Crain et al., 1976), and the 0.14 pg/nucleus found for the haploid sperm was similar to the 0.19 pg per Apis chromosome set determined by Jordan and Brosemer (1974). The Zeiss UMSP equipment used in our study allows extremely exact microphotometry of stechiometrically stained cell structures, as also obtained by Feulgen staining of nuclear DNA. In the honey bee, the spectrum of hemolymph proteins is dominated by the vitellogenin fraction in queens (Engels, 1974). But non-laying worker bees in queenright colonies also exhibit a high vitellogenin titer about five to six days after emergence (Engels et al., 1990). As recently shown by Barchuk et al. (2002), vitellogenin already appears in queens and workers towards the end of pupal development. Piulachs et al. (2003) detected vitellogenin mRNA in freshly moulted adult drones, similar to the results of Trenczek et al. (1989). We found that adult diploid drones also produce vitellogenin during the first week after emergence. There is some variation in hemolymph titer, but the average concentration is only about half that of haploid drones. Therefore, at an adult age of 5-6 days, the vitellogenin content of the hemolymph ranks Impact of diploid drones on the inclusive fitness of the bee colony According to the concept of the honey bee colony as a superorganism (Moritz and Southwick, 1992), a strong selection pressure eradicates features that reduce the inclusive fitness. Because of their failed reproductive capacity, the individual fitness of diploid drones is zero. Their removal (as larvae) is invariable, and several arguments support the evolutionary solution of early cannibalism of these ‘false males’ by the adult worker bees, in conformity with the kin selection theory (Hamilton, 1964). The impotent diploid drones would only ensure the transmission of their genes into the next generation by supporting the reproduction of their relatives, which is done by their worker-assisted suicide. Their own biomass is recycled by worker cannibalism, and the resources that would be required to further rear them are saved (Kukuk, 1992). This results in the establishment of early removal of the newly hatched diploid drone larvae, through which the brood comb area, as a limiting colonial investment, is immediately available again for oviposition by the queen (Ratnieks, 1990). As long as this diploid drone elimination is functioning well, the inclusive fitness of the colony is not much affected. On the other hand, no elimination signal would be needed to recognize advanced larval stages of diploid drones (Engels, 2004), which is evidenced by the ‘normalization’ of their pattern of hydrocarbons in the cuticular secretion of L2 and L3 larvae. Diploid drones - a mistaken creature into the bargain of Hymenopteran social evolution? The determination of the progeny’s sex by female control of egg fertilization prior to oviposition in the Hymenoptera is an advantage of the haplo-diploid mode of sex determination (Cook and Crozier, 1995). This allowed the evolution of social organization (Trivers and Hare, 1976), based on numerous female offspring and the division of labor between the reproductive and non-reproductive queen and worker castes. If the Hymenopteran ancestor had a diplo-diploid genotype, sex determination was probably mediated through sex chromosomes. Once haplo-diploidy evolved, probably by tetraploidization in the female karyotype (Fahrenhorst, 1977), this ancient mode of sexual genetics was replaced by single locus ‘complementary sex determination’ with a multiple allele system (Whiting, 1943). According to recent molecular analyses (Hasselmann et al., 2001), the Apis csd gene regions have different variabilities in the nucleotide sequence, indicating a continuing evolution of the gene structure (Beye et al., 2003). How the homozygous allele constellation at the scd locus is influencing the assumed differential splicing (Beye et al., 2002, 2003), as was found for Drosophila, is not yet clear. But the fact that male differentiation is the null-option whenever the primary signal for female development is missing, was clearly demonstrated (Beye et al., 2003). So diploid males are not erroneous output, but rather they are the inevitable consequence of Hymenopteran evolution (Page et al., 2002), only mitigated by a high-mating frequency of the reproducing females (Ratnieks, 1990; Boomsma and Ratnieks, 1996), as occurs in honey bees (Palmer and Oldroyd, 2000). The ultimate eugenic effect of diploid drone removal by the nursing workers is reasonable; the proximate solution of assisted suicide is not surprising since cannibalism is widespread in the animal kingdom and is regarded to be an influential agent in the evolution of social behavior (Elgar and Crespi, 1992). ACKNOWLEDGMENTS We thank Imkermeister Andreas Oelkrug for maintaining and preparing our experimental bee colonies, Wolfram Engels for helping prepare the figures, and Graeme Nicholson, Anja Bogdansky, Frank Mohr, Wolfram Simmendinger, Peter Rosenkranz, and Pia Aumeier for support and help with the GC-MS analyses, Giulia Santomauro for suggestions, and David De Jong for critically reading the manuscript. REFERENCES Adams J, Rothman ED, Kerr WE and Paulino ZL (1977). Estimation of the number of sex alleles and queen matings from diploid male frequencies in a population of Apis mellifera. Genetics 86: 583-596. Barchuk AR, Bitondi MMG and Simões ZLP (2002). Effects of juvenile hormone and ecdysone on the timing of vitellogenin appearance in hemolymph of queen and worker pupae of Apis mellifera. J. Insect Sci. 2: 1-8. Beye M, Härtel S, Hagen A, Hasselmann M et al. (2002). Specific developmental gene silencing in the honey bee using a homeobox motif. Insect Mol. Biol. 11: 527-532. Beye M, Hasselmann M, Fondrk MK, Page RE et al. (2003). The gene csd is the primary signal for sexual development in the honeybee and encodes an SR-type protein. Cell 114: 419-429. Bienefeld K, Mattausch A, Möller U and Pritsch G (1994). Ursache von Kannibalismus bei Arbeiterinnen der Honigbiene (Apis mellifera L.) an diploider Drohnenbrut. Verh. Dtsch. Zool. Ges. 87: 30. Bienefeld K, Mattausch A, Möller U and Pritsch G (2000). Kannibalismus-Substanz? - Ursachen für Brutlücken bei der Honigbiene? Dtsch. Bienenwirtsch 8: 20-21. Boomsma JJ and Ratnieks FLW (1996). Paternity in eusocial Hymenoptera. Philos. Trans. R. Soc. Lond. B Biol. Sci. 351: 947-975. Bull JJ (1983). Evolution of sex determining mechanisms. Benjamin/Cummings Publ. Comp. Inc., Menlo Park, CA, USA. Butcher RD, Whitfield WG and Hubbard SF (2000). Single-locus complementary sex determination in Diadegma chrysostictos (Gmelin) (Hymenoptera: Ichneumonidae). J. Hered. 91: 104-111. Camargo CA (1979). Sex determination in bees. XI. Production of diploid males and sex determination in Melipona quadrifasciata (Hymenoptera, Apidae). J. Apic. Res. 18: 77-84. Chaud-Netto J (1975). Sex determination in bees. II. Additivity of maleness genes in Apis mellifera. Genetics 79: 213-217. Cook J (1993). Sex determination in hymenoptera: a review of models and evidence. Heredity 71: 421-435. Cook J and Crozier RH (1995). Sex determination and population biology in the Hymenoptera. Tree 10: 281-286. Crain WR, Davidson EH and Britten RJ (1976). Contrasting patterns of DNA sequence arrangement in Apis mellifera (honeybee) and Musca domestica (housefly). Chromosoma 59: 1-12. Crozier RH (1971). Heterozygosity and sex determination in haplo-diploidy. Am. Nat. 105: 399-412. Dietz A (1975). Einfluß der “Kannibalismus-Substanz” diploider Drohnenlarven auf die Überlebensrate frischgeschlüpfter Arbeiterinnenlarven. 25th Int. Apimondia Congr. Grenoble, France, pp. 278-280. Dietz A and Lovins RW (1975). Studies on the “cannibalism substance” of diploid drone honey bee larvae. J. Ga. Entomol. Soc. 10: 314-315. Drescher W (1971). Selektionsversuche an einem Merkmal mit polygener Basis bei Apis mellifera L. 23rd Int. Apimondia Congr., Moscow, Russia, pp. 390-392. Drescher W (1972). Die Variation der 3. Cubitalzelle bei Carnicavölkern unter dem Einfluss der Selektion. Apimondia Int. Symposium, Paarungskontrolle und Selektion bei der Honigbiene. Lunz am See, Austria, pp. 79-82. Drescher W (1975). Wirkungsvolle Inzuchtsystemebei der Honigbiene: Zahl der Flügelhäkchen. Apimondia Int. Symposium, Paarungskontrolle und Selektion bei der Honigbiene. Lunz am See, Austria, pp.79-82. Drescher W and Rothenbuhler W (1964). Sex determination in the honey bee. J. Hered. 55: 90-96. Duchateau MJ, Hoshiba H and Velthuis HHW (1994). Diploid males in the bumble bee Bombus terrestris: sex determination, sex alleles and viability. Entomol. Exp. Appl. 71: 213-217. Dzierzon J (1845). Gutachten über die von Herrn Direktor Stöhr im ersten und zweiten Kapitel des General-Gutachtens aufgestellten Fragen. Eichstädter Bienenzeitung 1: 109-113, 119-121. Elgar MA and Crespi BJ (1992). Cannibalism: ecology and evolution among diverse taxa. Oxford University Press, Oxford, England. Engels W (1974). Occurrence and significance of vitellogenins in female castes of social Hymenoptera. Am. Zool. 14: 1229-1237. Engels W (2004). What bee larvae might tell their nurses? Proc. 8th IBRA Int. Conf. Tropical Bees, VI Encontro sobre Abelhas, Ribeirão Preto, SP, Brazil, pp. 18-26. Engels W and Fahrenhorst H (1974). Alters- und kastenspezifische Veränderungen der Haemolymph-Protein-Spektren bei Apis mellifica. W. Roux’ Arch 174: 285-296. Engels W, Kaatz H, Zillikens A, Simões ZLP et al. (1990). Honey bee reproduction: vitellogenin and caste-specific regulation of fertility. Adv. Invertebr. Reprod. 5: 495-502. Fahrenhorst H (1977). Nachweis übereinstimmender Chromosomenzahlen (n = 16) bei allen 4 Apis-Arten. Apidologie 8: 89-100. Garófalo CA (1973). Occurrence of diploid drones in a Neotropical bumblebee. Experientia 29: 726-727. Garófalo CA and Kerr WE (1975). Sex determination in bees. I. Balance between maleness and femaleness genes in Bombus atratus Franklin. Genetica 45: 203-209. Gonçalves LS (1972). Untersuchung über das morphologische Merkmal Zahl der Flügelhäkchen bei Apis mellifera. Apimondia Int. Symposium, Paarungskontrolle und Selektion bei der Honigbiene. Lunz am See, Austria, pp. 83-86. Gonçalves LS (1976). Seleção direcional em duas linhagens endocruzadas de Apis mellifera L. Livre Doc. thesis, USP, Ribeirão Preto, SP, Brazil. Hamilton WD (1964). The genetical evolution of social behaviour. II. J. Theor. Biol 7: 1-52. Hasselmann MU and Beye M (2001). Narrow down the sex locus ene of the honeybee (Apis mellifera). Zoologie 104: 12. Hasselmann M and Beye M (2004). Signatures of selection among sex-determining alleles of the honey bee. Proc. Natl. Acad. Sci. USA 101: 4888-4893. Heinrichs V, Ryner LC and Baker BS (1998). Regulation of sex-specific selection of fruitless 5' splice sites by transformer and transformer-2. Mol. Cell. Biol. 18: 450-458. Hepperle C and Hartfelder K (2001). Differentially expressed regulatory genes in honey bee caste development. Naturwissenschaften 88: 113-116. Herrmann M and Engels W (2005). In vitro rearing of honey bee (Apis mellifera) larvae with semiartificial diets and a feeding apparatus. Entomol. Gen. 25: ( in press). Hoshiba H, Okada I and Kusanagi A (1981). The diploid drone of Apis cerana japonica and its chromosomes. J. Apic. Res. 20: 143-147. Jordan RA and Brosemer RW (1974). Characterization of DNA from three bee species. J Insect Physiol. 20: 2413-2520. Kerr WE (1969). Some aspects of the evolution of social bees (Apidae). Evol. Biol. 3: 119-175. Kerr WE (1997). Sex determination in bees (Apinae, Meliponinae) and its consequences. Braz. J. Genet. 20: 601-612. Kerr WE and Nielsen RA (1967). Sex determination in bees (Apinae). J. Apic. Res. 6: 3-9. Kühnert M (1991). Demonstration of new techniques using instrumental insemination. Apiacta 26: 2-7. Kukuk PF (1992). Cannibalism in social bees. In: Cannibalism: Ecology and evolution among diverse taxa (Elgar MA and Crespi BJ, eds.). Oxford University Press, Oxford, New York, Tokyo, pp. 214-237. Laurell CB (1965). Antigen-antibody crossed electrophoresis. Anal. Biochem. 10: 358-361. Leys R (1994). Diploid andrenid males can be recognized using morphometric data. Insectes Soc. 41: 458-462. MacKensen O (1951). Viability and sex determination in the honey bee (Apis mellifera). Genetics 36: 500-509. MacKensen O (1955). Further studies on a lethal series in the honeybee. J. Hered. 46: 72-74. Michener CD (2000). Bees of the world. John Hopkins University Press, Baltimore, MD, USA. Mirsky AE and Ris H (1951). The desoxyribonucleic acid content of animal cells and its evolutionary significance. J. Gen. Physiol. 34: 451-462. Moritz RFA and Southwick EE (1992). Bees as superorganisms. An evolutionary reality. Springer Berlin, Heidelberg, New York. Nachtsheim H (1913). Cytologische Studien über die Geschlechtsbestimmung bei der Honigbiene (Apis mellifica L.). Arch. Zellforsch. 11: 170-239. Page Jr RE, Gadau J and Beye M (2002). The emergence of Hymenopteran genetics. Genetics 160: 375-379. Palmer KA and Oldroyd BP (2000). Evolution of multiple mating in the genus Apis. Apidologie 31: 235-248. Paulcke W (1899). Zur Frage der parthenogenetischen Entstehung der Drohnen (Apis mellifica). Anat. Anz. 16: 185-187. Paxton RJ, Weißschuh N, Engels W, Hartfelder K et al. (1999). Not only single mating in stingless bees. Naturwissenschaften 86: 143-146. Paxton RJ, Bego LR, Shah MM and Mateus S (2003). Low mating frequency of queens in the stingless bee Scaptotrigona postica and worker maternity of males. Behav. Ecol. Sociobiol. 53: 174-181. Petrunkewitsch A (1901). Die Richtungskörper und ihr Schicksal im befruchteten und unbefruchteten Bienenei. Zool. Jahrb. Abt. Anat. Ontog. Tiere 14: 573-608. Piulachs MD, Guidugli KR, Barchuk AR, Cruz J et al. (2003). The vitellogenin of the honey bee, Apis mellifera: structural analysis of the cDNA and expression studies. Insect Biochem. Mol. Biol. 33: 459-465. Polaczek B, Neumann P, Schricker B and Moritz RFA (2000). A new, simple method for rearing diploid drones in the honeybee (Apis mellifera L.). Apidologie 31: 525-530. Ratnieks F (1990). The evolution of polyandry by queens in social hymenoptera: the significance of the timing of removal of diploid males. Behav. Ecol. Sociobiol. 26: 343-348. Rothenbuhler WC (1957). Diploid male tissue as new evidence on sex determination in honeybees. J. Hered. 48: 160-168. Ruttner F (1975). Die instrumentelle Besamung der Bienenkönigin. Apimondia Publishing House, Bucharest, Romania. Santomauro G and Engels W (2002). Sexing of newly hatched live larvae of the honey bee Apis mellifera allows the recognition of diploid drones. Apidologie 33: 283-288. Santomauro G, Oldham NJ, Boland W and Engels W (2004). Cannibalism of diploid drone larvae in the honeybee (Apis mellifera) is released by odd pattern of cuticular substances. J. Apic. Res. 43: 69-74. Saul GB, Whiting PW, Saul SW and Heidner CA (1965). Wild-type and mutant stocks of Mormoniella. Genetics 52: 1317-1327. Schutt C and Nothiger R (2000). Structure, function and evolution of sex-determining systems in Dipteran insects. Development 127: 667-677. Siebold CTE (1856). Wahre Parthenogenese bei Schmetterlingen und Bienen. Engelmann, Leipzig, Germany, p. 144. Skowronek W, Kruk C and Loc K (1995). The insemination of honey bees with diluted semen. Apidologie 26: 487-493. Snodgrass RE (1956). Anatomy of the honey bee. Comstock Publishing Associates, Ithaca, London. Tarelho ZFS (1973). Contribuição ao estudo citogenético dos Apoidea. Doctoral thesis, University of São Paulo, Ribeirão Preto, SP, Brazil. Tozetto S. de O, Rachinsky A and Engels W (1997). Juvenile hormone promotes flight activity in drones (Apis mellifera carnica). Apidologie 28: 77-84. Trenczek T and Engels W (1986). Occurrence of vitellogenin in drone honeybees (Apis mellifica). Int. J. Invertebr. Reprod. Develop. 10: 307-311. Trenczek T, Zillikens A and Engels W (1989). Developmental patterns of vitellogenin haemolymph titre and rates of synthesis in adult drone honeybees (Apis mellifera). J. Insect Physiol. 35: 475-481. Trivers RL and Hare H (1976). Haplodiploidy and the evolution of the social insect. Science 191: 249-263. Tyler J (2004). BCM completes honeybee genome sequence. http://public.bcm.tmc.edu/pa/genome-honeybee.htm#top. Accessed January 2004. Whiting PW (1943). Multiple alleles in complementary sex determination of Habrobracon. Genetics 28: 365-382. Whiting PW and Whiting AR (1925). Diploid males from fertilized eggs in Hymenoptera. Science 62: 437-438. Woyke J (1962). The hatchability of “lethal eggs” in a two sex-allele fraternity of honeybees. J. Apic. Res. 1: 6-13. Woyke J (1963a). Drone larvae from fertilised eggs of the honeybee. J. Apic. Res. 2: 19-24. Woyke J (1963b). What happens to diploid drone larvae in a honeybee colony? J. Apic. Res. 2: 73-75. Woyke J (1963c). Drones from fertilized eggs and the biology of sex determination in the honeybee. Bull. Acad. Polon. Sci. Cl. 11: 251-254. Woyke J (1963d). The behaviour of queens inseminated artificially in different manner. 19th Int. Beekeep. Congr. Apimondia, Prague, Czech Republic, pp. 702-703. Woyke J (1965). Genetic proof of the origin of drones from fertilized eggs of the honeybee. J. Apic. Res. 4: 7-11. Woyke J (1967). Diploid drone substance - cannibalism substance. 21st Int. Beekeep. Congr. Apimondia, Maryland, USA. Woyke J (1969a). A method of rearing diploid drones in a honeybee colony. J. Apic. Res. 8: 65-74. Woyke J (1969b). Rearing diploid drones on royal jelly or bee milk. J. Apic. Res. 8: 169-173. Woyke J (1973). Reproductive organs of haploid and diploid drone honeybees. J. Apic. Res. 12: 35-51. Woyke J (1974). Genetic balance, heterozygosity and inheritance of size of testes in diploid drone honeybees. J. Apic. Res. 13: 77-91. Woyke J (1975). DNA content of spermatids and spermatozoa of haploid and diploid drone honeybees. J. Apic. Res. 14: 3-8. Woyke J (1977). Cannibalism and brood-rearing efficiency in the honey bee. J. Apic. Res. 16: 84-94. Woyke J (1978a). Comparative biometrical investigation on diploid drones of the honeybee. II. The thorax. J. Apic. Res. 17: 195-205. Woyke J (1978b). Comparative biometrical investigation on diploid drones of the honeybee. III. The abdomen and weight. J. Apic. Res. 17: 206-217. Woyke J (1979). Sex determination in Apis cerana indica. J. Apic. Res. 118: 122-127. Woyke J (1980). Evidence and action of cannibalism substance in Apis cerana indica. J. Apic. Res. 19: 6-16. Woyke J and Skowronek W (1974). Spermatogenesis in diploid drones of the honeybee. J. Apic. Res. 183-190. |
|