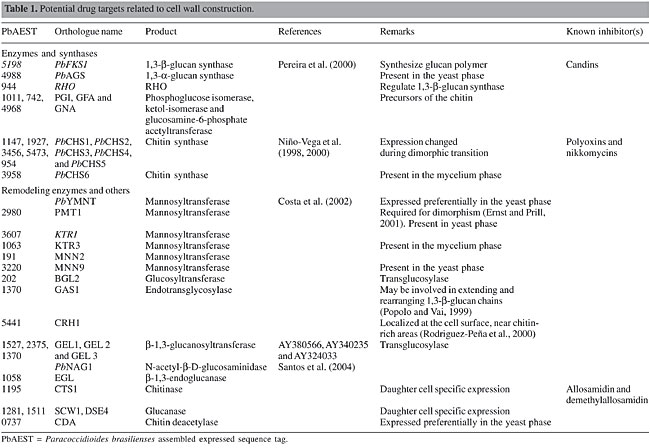
ABSTRACT. The rise in antifungal resistance, observed as a result of the increasing numbers of immunocompromised patients, has made the discovery of new targets for drug therapy imperative. The description of the Paracoccidioides brasiliensis transcriptome has allowed us to find alternatives to refine current therapy against paracoccidioidomycosis. We used comparative analysis of expressed sequence tags to find promising drug targets that have been addressed in other pathogens. We divided the analysis into six different categories, based on the involvement of the targeted mechanisms in the cell: i) cell wall construction, ii) plasma membrane composition, iii) cellular machinery, iv) cellular metabolism, v) signaling pathways, and vi) other essential processes. Through this approach, it has been possible to infer strategies to develop alternative drugs against this pathogen. Key words: Drug targets, Fungi, Paracoccidioides brasiliensis INTRODUCTION The incidence and severity of mycoses have grown to alarming levels worldwide. Patients with immune deficiency have contributed to this scenario since they are more susceptible to unconventional, more aggressive forms of classical mycoses, and they also tend to harbor resistant varieties of the pathogens. Antifungal agents currently available for treatment of systemic mycoses include four groups of drugs, namely Polyenes (amphotericin B), Fluoropyrimidines (flucytosines), Azoles (ketoconazole, fluconazole, itraconazole), and Echinocandins (caspofungin and mycafungin). More recently, compounds from the Allylamine group (terbinefine) have shown high synergy with drugs from the Azole group, but even this drug association is unable to treat some fungal infections adequately. In addition, antifungals cause serious side effects, such as nephrotoxicity. In this context, the search for alternative therapies and the development of more specific antifungal drugs are urgently needed (Georgopapadakou and Walsh, 1996; Wills et al., 2000; Kontoyiannis and Lewis, 2002). Some strategies have been employed to develop antifungal drugs. One of them is the classical method, in which screening is done in vitro with a great variety of compounds, either extracted from plants and animals or synthesized in vitro (Wills et al., 2000). The refinement of classical drugs or of administration methods is a promising alternative to optimize pharmacological action at the site of infection or to increase specificity. More recently, nanotechnology has been envisaged to administer safe and effective low dosages of drugs, diminishing side effects and complications for the patients, such as amphotericin B (Fukui et al., 2003; Sobue and Sekiguchi, 2004). In this era of genomic science, genome sequencing and bioinformatics drive the discovery of new antifungal targets. We have made efforts to identify such targets, or at least candidates, in Paracoccidioides brasiliensis, based on transcriptome analysis and on sites of action of antimycotic drugs already described in the literature, either for P. brasiliensis or for other fungal pathogens. CELL WALL SYNTHESIS The cell wall of human pathogenic fungi is in close contact with the host and serves as a barrier against host defense mechanisms. Human cells are ineffective in degrading cell wall polysaccharides of P. brasiliensis, as shown by ultrastructural studies of yeast cells ingested by activated macrophages, which revealed empty cells with intact walls (Brummer et al., 1990). The cell wall has an essential role in the pathobiology of P. brasiliensis, since it is directly linked to the morphogenetic changes associated with the fungal life cycle. The cell wall remains to be explored in detail as a target for antifungals (Selitrennikoff and Nakata, 2003). Genes involved in cell wall metabolism in P. brasiliensis have been isolated, and many others were identified in the transcriptome (Table 1). Some are differentially expressed, such as chitin synthases (Niño-Vega et al., 2000), hydrophobins (Albuquerque et al., 2004), mannosyltransferase-Pbymnt (Costa et al., 2002), and chitin deacetylase - cda (Felipe et al., 2005) and are candidates for drug targets.
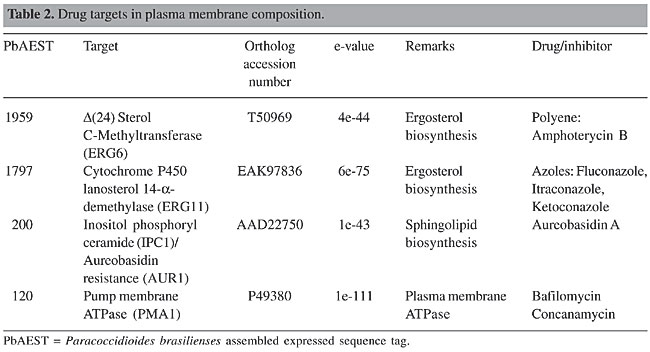
Synthases 1,3-b-glucan synthase In P. brasiliensis, 1,3-b-glucan synthase requires uridine diphosphate (UDP)-glucose as the preferred nucleotide precursor to the in vitro synthesis of b-glucan (San-Blas, 1979). Only one homolog to 1,3-b-glucan synthase, PbFKS1, has been cloned from this fungus (Pereira et al., 2000). The presence of putative regulatory signals suggests flexible and possibly complex control mechanisms for the expression of PbFKS1. Although the UDP-glucose-binding motif was not found, domain analogs to cellulose synthase, proposed by Kelly et al. (1996), are present in PbFKS1. Analyses suggest that PbFks1p is localized within the cytoplasmic membrane and possesses a catalytic cytoplasmic domain between two transmembrane regions. PbFks1p seems to assemble the phosphorylated glucan polymer and simultaneously extrude it out of the membrane, since a phosphotransferase system-phosphoryl carrier protein component phosphorylation site motif has been found. 1,3-b-glucan synthase is regulated by the RHO GTPases, which is a multifunctional regulator related to numerous proteins (Douglas, 2001). The role for Rho1p in the regulation of 1,3-b-glucan synthase has been described in pathogenic fungi, such as Candida albicans (Kondoh et al., 1997), Aspergillus fumigatus (Beauvais et al., 2001) and Cryptococcus neoformans (Tanaka et al., 1999). Two RHO genes were identified in the transcriptome of P. brasiliensis. RHO1 is a key regulator of 1,3-b-glucan synthase in budding and fission yeasts and in C. albicans (Kondoh et al., 1997). In Schizosaccharomyces pombe, Rho2 GTPase functions as a regulatory factor of 1,3-a-glucan synthase (Calonge et al., 2000). Echinocandins disturb the membrane and cause a loss of the enzymatic activity of 1,3-b-glucan synthase without any direct binding to the catalytic site, thus compromising the structure and osmolarity of the cell wall (Klepser, 2003). Three echinocandins are currently in clinical development: caspofungin (Merck & Co., Inc., Rahway, NJ, USA), micafungin (Fujisawa Inc., Deerfield, IL, USA), and anidulafungin (formerly LY303366; Versicor Inc., Freemont, CA, USA). The 1,3-b-glucan synthase inhibitors papulacandin B, micafungin and cilofungin are more active against the mycelial form of P. brasiliensis (Dávila et al., 1986; Hanson and Stevens, 1989; Nakai et al., 2003). A new generation of semi-synthetic candins is emerging (Tkacz and DiDomenico, 2001) and natural-product screening by a series of new methods has also identified other 1,3-b-glucan synthase inhibitors (Onishi et al., 2000; Barrett, 2002). 1,3-a-glucan synthase 1,3-a-glucan synthase is proposed as a virulence factor in P. brasiliensis (San-Blas et al., 1977), as well as in Blastomyces dermatitidis (Hogan and Klein, 1994) and Histoplasma capsulatum (Klimpel and Goldman, 1988). We identified the 1,3-a-glucan synthase gene, Ags2, in the yeast phase of the P. brasiliensis transcriptome. Few 1,3-a-glucan synthase genes have been isolated to date. Aspergillus fumigatus mutants show a slight reduction in the growth rate and in the concentration of cell wall 1,3-a-glucan synthase (Bernard and Latgé, 2001). To our knowledge, no inhibitors of 1,3-a-glucan synthase have been identified. The search for these inhibitors is very important since the yeast pathogenic phase presents 1,3-a-glucan as an almost exclusive (95% of the cell wall) glucan polymer (Kanetsuna et al., 1972). Chitin synthase Membrane-bound chitin synthase catalyses the polymerization of GlcNAc (N-acetyl-b-D-glucosaminidase) from cytosolic UDP-GlcNAc into polysaccharide chains that are extruded to the cell wall (Ruiz-Herrera, 1992; Gooday, 1995). Chitin synthesis in fungi is a complex process (Horiuchi and Takagi, 1999), regulated by multigene families and involved in distinct physiological processes (Cabib, 1991; Gaughran et al., 1994). Chitin synthases are inhibited by nucleoside tri- and dipeptide molecules, polyoxins and nikkomycins, respectively. These inhibitors, structurally analogous to UDP-GlcNAc, are transported by peptide permeases into the cell, where they probably act by binding to the catalytic site of the chitin synthase. Transport failure and low permeability of the inhibitors result in resistance (Ruiz-Herrera and San Blas, 2003; Gozalbo et al., 2004). Although it is possible to express CHS genes in a heterologous host (Silverman et al., 1988; Bulawa et al., 1986), these transmembrane proteins are not soluble recombinant molecules amenable to direct high-throughput screening. Studies of the molecular mechanism of chitin polymerization (Ruiz-Herrera and San Blas, 2003) and crystallization (Ruiz-Herrera, 1992) may reveal new drug targets in the future. Five chitin synthases were identified by PCR amplification of conserved CHS gene domains in P. brasiliensis. Despite the fact that yeast cells contain more chitin than do hyphae, the levels of mRNAs for PbCHS1, PbCHS2, PbCHS4, and PbCHS5 are higher in the mycelium (Niño-Vega et al., 2000), suggesting that post-transcriptional regulation of CHS gene expression is important for morphogenesis. We have identified, by means of transcriptome analysis, a new chitin synthase, CHS6, differentially expressed in the mycelium phase of P. brasiliensis. Phosphoglucose isomerase, glutamine fructose-6-phosphate amidotransferase and glucosamine-6-phosphate acetyltransferase are critical enzymes in the synthesis of cell wall precursors. Since low levels of these precursors result in cell wall abnormality, leading to fungal cell death, these enzymes, also present in the P. brasiliensis transcriptome, are potential targets for antifungal drugs (Selitrennikoff and Nakata, 2003). Remodeling enzymes Mannosyltransferase Some proteins present in the cell wall are glycosylated on their serine or threonine amino acids by the addition of mannose residues. Mannosyltransferases, Pmt1p and Mnt1p, are important for cell wall structure, adhesion and virulence (Gozalbo et al., 2004). In addition PMT1 is required to dimorphism (Ernst and Prill, 2001). Another protein involved in the synthesis of N-linked outer chain mannans is codified by the MNN9 gene. Disruption mutants for this gene exhibit phenotypes with characteristic defects in cell wall biosynthesis and/or assembly (Wills et al., 2000). Benanomicins and pradimicins bind selectively to the terminal D-mannosides of mannans in the cell wall and cell membrane of fungal cells in a calcium-dependent manner, resulting in insoluble complexes (Gozalbo et al., 2004). The PMT1, MNN2, KTR1, KTR3, MNN9, and PbYMNT1 genes identified in P. brasiliensis transcriptome are promising targets for future antifungal therapy. Cross-linking among cell wall components Cell wall polymers are linked differentially to form the final architecture responsible for different morphologies in fungi (Klis et al., 2001). Enzymes involved in the integration of 1,3-b-glucans have been described. They are Gas1p, an endotransglycosylase that may be involved in extending and rearranging 1,3-b-glucan chains (Popolo and Vai, 1999), Bgl2p, an endotransglycosylase that introduces intrachain 1,6-b-linkages (Goldman et al., 1995), Crh1p (Rodrigues-Peña et al., 2000) and the homolog to GPI-anchored 1,3-b-glucanosyltransferases Gel1p (Beauvais and Latgé, 2001). Genes that encode these enzymes were identified in the P. brasiliensis transcriptome. Transglycosidases play an active role in cell wall synthesis and in fungal morphogenesis (Beauvais and Latgé, 2001). Since enzymes responsible for the branching of 1,3-b-glucan or the linkage of chitin to 1,3-b-glucan are active in the periplasmic space and involve essential biosynthetic steps, they present good candidates for drug development. To our knowledge, no inhibitors of these enzymes have been identified. Hydrolases Hydrolytic enzymes have different roles in the morphogenetic events (Wessels, 1988). In contrast to the other fungal b-1,3-endoglucanases reported in the literature, which are exocellular, the first cell wall-associated fungal b-1,3-endoglucanase was identified in A. fumigatus (Mouyna et al., 2002). In addition, several cell wall-associated and secreted chitinases have been identified (Mellor et al., 1994; Hearn et al., 1998). Inhibition of chitinases by antibiotics, such as allosamidin or demethylallosamidin, leads to the formation of clumps, as daughter cells are unable to separate from mother cells during budding (Gozalbo et al., 2004). The chitinase and b-1,3-endoglucanase genes were identified in the transcriptome of P. brasiliensis. Our group has cloned and characterized PbNAG1, encoding an NAG, a glycosyl hydrolase family 20 protein (Santos et al., 2004). Another predicted NAG gene, PbNAG2, is currently under study. PLASMA MEMBRANE COMPOSITION The fungal plasma membrane is the most important target for antifungal drugs in current therapy. It is based on the fact that the plasma membrane acts as the main interface between the cell and the environment of all organisms. Disturbances in the environment are perceived by the cell across transmembrane proteins, and a cascade of signals is started to allow for adaptation. In this context, the transduction of signals will culminate in membrane composition alterations to adapt to different situations (Georgopapadakou and Walsh, 1996; Wills et al., 2000). The cell has the ability to adapt to different levels of pressure, pH variation and mechanical stress. Ergosterol and sphingolipids are responsible for the shape and rigidity of the plasma membrane. If some steps of their biosynthetic pathways are blocked, the intermediates may accumulate inside the cell and promote an excessive stiffness in the membrane (Wills et al., 2000; Theis and Stahl, 2004). Pump-mediated efflux confers the cell with a way to exchange ions and nutrients, granting optimal pH and energy reserves. Depletion in the mechanisms of pump efflux may impair synthesis of important cellular components, including cell wall precursors (Luo et al., 1999; Theis and Stahl, 2004). Since some drugs can affect the integrity of the cell by forming pores in the plasma membrane and promoting the leakage of crucial components, they constitute natural candidate antimycotics. Ergosterol The ergosterol biosynthetic pathway is the best-explored target of current therapy. The final product is ergosterol itself, which is the main exclusive fungal sterol, and it localizes to the cytoplasmic membrane. Some enzymes participating in ergosterol biosynthesis are not present in human cells. Different classes of antifungal agents target components of ergosterol biosynthesis (Georgopapadakou and Walsh, 1996; Wills et al., 2000; Odds et al., 2003; Onyewu et al., 2003). The polyene antimycotic, amphotericin B, is an important antifungal agent that acts only against ergosterol and not against mammalian cholesterol synthesis (Odds et al., 2003). Despite its fungicidal effect, it has serious side effects, such as nephrotoxicity. With the advent of nanotechnology, some formulations of this drug have been developed to overcome these undesired reactions. This drug has a large spectrum of action and is indicated for treatment against Candida spp and other pathogenic fungi, including P. brasiliensis (Wills et al., 2000; Odds et al., 2003; Onyewu et al., 2003). Erg6 encodes an S-adenosylmethionine:D24-methyltransferase and its disruption in C. albicans and S. cerevisiae results in increased resistance to polyenes and decreased ergosterol content (Young et al., 2003). The azoles act in several steps of ergosterol biosynthesis by inhibiting enzymes in this pathway, which varies among the different fungal species. The main target for this class of drug is cytochrome P450 lanosterol 14-a-demethylase, which is encoded by Erg11 and also has an iron protoporphyrin moiety at the active site, where the drug binds. Ketoconazole, itraconazole and fluconazole have been used in antifungal therapy. They basically prevent the demethylation of lanosterol, resulting in defective synthesis or in the depletion of ergosterol (Wills et al., 2000; Smith and Edlind, 2002; Odds et al., 2003; Theis and Stahl, 2004). Paracoccidioides brasiliensis presents ERG6 and ERG11 in its transcriptome (Table 2). Although some enzymes of the ergosterol pathway are not exclusively fungal, they can be exploited as potential targets for drug development.
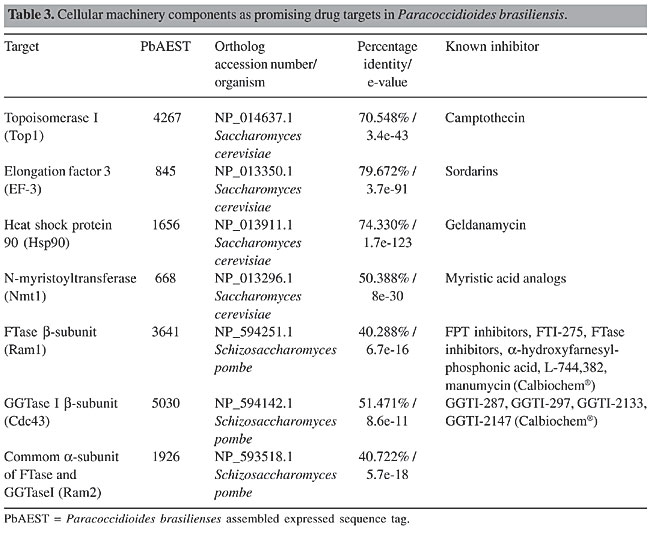
Sphingolipids The sphingolipid pathway is considered a potential target for the development of new antimycotics. Sphingolipids are involved in several intracellular responses, including heat shock-induced stress, endocytosis, apoptosis, and signal transduction. They have important roles in the maintenance of the plasma membrane of eukaryotic cells, and they are thus required for cellular growth and viability (Luberto et al., 2001; Obeid et al., 2002; Thevissen et al., 2004). Although sphingolipids are present in both mammalian and yeast membranes, some differences are found between the respective synthetic pathways (Obeid et al., 2002). The most abundant sphingolipid in the plasma membrane is inositol phosphoryl ceramide (IPC), synthesized by IPC synthase; the corresponding gene IPC1 (also named AUR1) has also been identified in the P. brasiliensis transcriptome. This enzyme transfers phosphorylinositol from phosphatidylinositol to the 1-hydroxy group of phytoceramide to form IPC (Zhong et al., 2000; Wills et al., 2000). IPC1/AUR1 is absent from mammalian cells, although both mammals and yeast use the same substrate to produce sphingomyelin and inositol phosphoryl ceramide, respectively (Wills et al., 2000; Heung et al., 2004). In C. neoformans, Ipc1 is involved in the production of melanin, a virulence factor and a component used to protect the fungus against the immunological host response (Heung et al., 2004). Down-regulation of this gene causes a deficient response at low pH, whereby, as can be inferred in an in vivo model, virulence is diminished (Luberto et al., 2001). Some studies have demonstrated that IPC is essential for survival of some fungi. Sensibility tests are being carried out with a variety of compounds to find inhibitors of IPC synthase. One prominent compound is the Aureobasidin A, a cyclic nonadepsipeptide, extracted from Aureobasidium pullulans (Zhong et al., 2000; Wills et al., 2000), which is active against C. albicans, S. cerevisiae and C. neoformans at lower concentrations when compared to other antifungals (Zhong et al., 2000). As IPC synthases are not present in human cells, the use of IPC inhibitors as antifungal agents is a good possibility for the therapy of systemic mycoses in humans. The Ipc1 gene has been found in the P. brasiliensis transcriptome (Table 2). Previous studies in our laboratory have demonstrated that IPC1 is a promising target for drug development against P. brasiliensis. Proton ATPases Plasma membrane ATPase plays important roles in the maintenance of cell homeostasis by regulation of pH through ionic exchange. This mechanism creates a membrane potential and an electrochemical gradient that allows the uptake of ions and nutrients required for cellular physiology. The proton pump mechanism enables the cell to tolerate different pH gradients (Luo et al., 1999; Wills et al., 2000; Burghoorn et al., 2002; Lee et al., 2002; Wang and Chang, 2002; Pizzirusso and Chang, 2004). Recently, studies have demonstrated that bafilomycin inhibits ATPase activity with high specificity and potency. Bafilomycin and concanamycin, antibiotics with similar structures, have been used as potent anti-tumor agents. Experiments with mutations in different sites of ATPase protein in strains of Neurospora crassa caused this fungus to overcome the toxic effects of concanamycin (Bowman and Bowman, 2002). We identified the gene PMA1 in the P. brasiliensis transcriptome (Table 2); it is known to encode the plasma membrane ATPase in other fungi, including S. cerevisiae. Although PMA1 is also present in the human transcriptome, several domains are exclusive to fungi, and gene deletion has been demonstrated to be lethal for some of those microorganisms. ATPases are promising targets for the development of antimycotics, and the differences between fungal and mammalian proteins need to be further investigated (Georgopapadakou and Walsh, 1996; Wills et al., 2000). CELLULAR MACHINERY Several components of cellular machinery were demonstrated in other fungi as potential drug targets, and promising inhibitors to them are being tested (Table 3). Topoisomerase 1 (Top1) and elongation factors belong to the nuclear machinery, where they are key components in DNA-RNA-protein synthesis. Moreover, the heat shock protein Hsp90, which is required for proper protein refolding upon thermal stress, and three different proteins involved in post-translational modifications, N-myristoyltransferase (NMT), farnesyltransferase (FTase) and geranylgeranyltransferase (GGtase) I, were examined by our project.
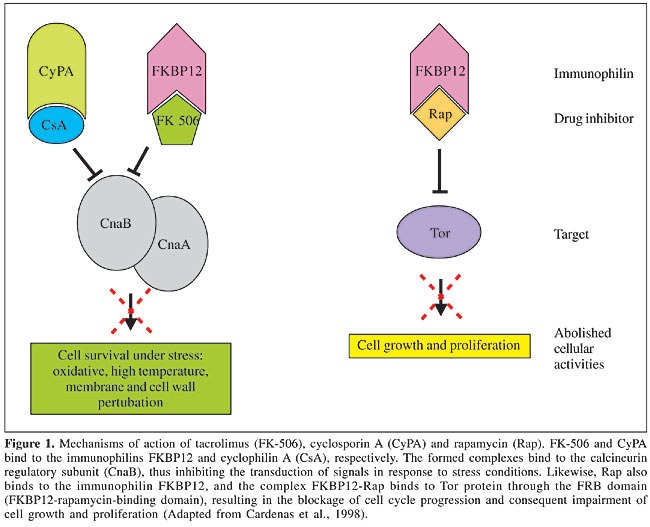
Topoisomerase Topoisomerases are enzymes that act on chromosome replication, transcription, recombination, and segregation processes by controlling the topological state of DNA. Their classification is related to how they cleave the DNA strand: TOP1 cleaves and rearranges one DNA strand, while TOP2 can cleave both DNA strands (Stewart et al., 1998). Topoisomerase-specific inhibitors, for example camptothecin or its analog topothecan, stabilizes the complex topoisomerase-DNA, leading to DNA damage and cell death (D’Arpa and Liu, 1989). TOP1 has been demonstrated to be essential and a virulence factor for some fungi, and its deletion in C. albicans induces slow cellular growth and aberrant cell morphology (Fostel et al., 1992; Jiang et al., 1997). The fungal TOP 1 gene has a considerable number of regions that are not present in the human transcriptome (Stewart et al., 1996). Selective inhibition of fungal (C. albicans) Top1p catalytic activity when compared to the human enzyme has already been observed using the aminocatechol A-3253 (Fostel and Montgomery, 1995). We found the Top1 in the P. brasiliensis transcriptome, with identities of 90, 80 and 58% with A. nidulans (EAA66126.1), N. crassa (XP_331510.1) and human (CAA18536.1), respectively, indicating that the differences between human and fungal Top1p can be explored by drug design. Elongation factors Elongation factors required for protein synthesis are being focused on as potential drug targets in fungi. Some sordarin derivatives, such as GM 222712 and GM 237354, have shown in vitro activity against a wide range of pathogenic fungi, including Aspergillus spp, Candida spp, C. neoformans, and other filamentous fungi (Andriole, 2000; Wills et al., 2000). Sordarins act by blocking the elongation cycle at the initial steps of translocation, prior to GTP hydrolysis, thus preventing the formation of peptidyl-[(3)H] puromycin on polysomes in C. albicans. Elongation factor 3 is a soluble form required for fungal translation machinery that is absent from other organisms, including humans, and hence a very attractive target for antifungal therapy (Wills et al., 2000). It was also identified in the P. brasiliensis transcriptome. Hsp90 Hsps are highly conserved among different organisms. Hsp90 is a heat shock protein of approximately 90 kDa that is required for the refolding or degradation of cellular proteins upon heat shock, and it appears to be linked to the immune response against fungal pathogens (Matthews et al., 1998). The P. brasiliensis Hsp90 is differentially expressed by the pathogenic yeast phase (Felipe et al., 2005). Geldanamycin is a benzoquinone ansamycin antibiotic produced by Streptomycetes that binds with high specificity within the ADP/ATP binding pocket of Hsp90, thereby inhibiting its function. Cells lacking hsp90 functions are severely compromised and cannot progress through the cell cycle (Cardenas et al., 1998; Piper, 2001). Despite the high conservation of Hsp90 between yeast and humans, and the evidence that geldanamycin is not toxic to wild-type yeast strains (Cardenas et al., 1998), development of specific antifungal inhibitors of Hsp90 deserves attention due to its role in P. brasiliensis dimorphism and pathogenesis. N-myristoyltransferase NMT catalyses the transfer of myristate from Co-enzyme A to the amino-terminal Gly residue of a number of cellular proteins involved in signal transduction pathways (Nagarajan et al., 1997). This process is observed only in eukaryotic cells. Genetic studies have shown that NMT is essential for the viability of the several fungal pathogens that cause systemic infection in immunosuppressed individuals (Lodge et al., 1994). Comparative analysis of the peptide substrate specificities of human and yeast NMT revealed distinct differences in peptide recognition, despite the high degree of conservation in the acyl-Co-enzyme A substrate specificities (Rocque et al., 1993). These differences in peptide recognition provide an opportunity to develop species-specific enzyme inhibitors that act as antifungals. Moreover, myristic acid analogs proposed as inhibitors of NMT were tested in vitro for activity against the budding yeasts S. cerevisiae, C. albicans, C. neoformans, and the filamentous fungus Aspergillus niger (Parang et al., 1996). In addition, in 1997, Nagarajan et al. described the development of a potent peptidomimetic class-specific inhibitor for the C. albicans NMT. In the P. brasiliensis transcriptome analysis we identified the gene Nmt1, which codifies glycylpeptide N-tetradecanoyltransferase, commonly known as NMT. Prenyltransferases: farnesyltransferase and geranylgeranyltransferase Prenylation is a post-translational protein modification process, in which there is an addition of a prenyl group - farnesyl diphosphate or geranylgeranyl diphosphate, derived from ergosterol biosynthesis, to the C-terminus of the protein, resulting in its correct localization to cell membranes. This process is mediated by prenyltransferases (PTFs), such as FTase, and GGTases I and II. Prenylated proteins participate in a variety of cellular functions, such as control of cell growth, differentiation, cytokinesis, membrane trafficking, and signal transduction. Proteins that are prenylated include small GTP-binding proteins, lipopeptide pheromones, nuclear lamins, and trimeric G-proteins (Schafer and Rine, 1992). Many of these GTPases also participate in cell signaling pathways, which are likely to be involved in the pathogenesis of C. albicans and C. neoformans (McGeady et al., 2002; Vallim et al., 2004). All PTFs in yeasts and mammals consist of a- and b-subunits. The a-subunit is encoded by the RAM2 gene and is shared between FTase and GGTase I; the b-subunits of FTase and GGTase I are encoded by RAM1 and CDC43, respectively. In the P. brasiliensis transcriptome analysis, we identified all of these subunits, suggesting the ability of this pathogen to use both PTFs in its morphogenesis, in contrast with C. neoformans, for which just the FTase has been identified to date (Vallim et al., 2004). Compounds that inhibit protein prenylation have been developed and studied, as potential agents to treat human malignancies, mainly due to the observation that activated Ras mutations are associated with a significant number of human cancers. Initial trials indicated that inhibiting prenylation could result in a reduction in the growth rate of some tumor lines (Kohl et al., 1995). More recently, these compounds were assayed in vitro in the opportunistic pathogens, C. albicans and C. neoformans, to assess the effect of blocking the prenylation process as a way to avoid differentiation and pathogenesis (McGeady et al., 2002; Vallim et al., 2004). The results are very promising, in that prenylation inhibitors blocked the serum-induced conversion from the yeast to the filamentous form (this latter being closely related to pathogenesis) in C. albicans and impaired hyphal differentiation in C. neoformans. Moreover, the poor similarity between fungal and human PTFs makes them very attractive drug targets to be evaluated in P. brasiliensis. SIGNALING PATHWAYS Pathogenic organisms have co-opted conserved signaling pathways to sense and respond to the host harsh conditions and to activate the genes required for virulence and pathogenesis. For this reason, many studies are focusing on signaling cascade components as possible targets for antifungal therapy. In our biological model, P. brasiliensis, several signaling pathways have been described; nonetheless, only calcineurin and TOR will be discussed below. Calcineurin Calcineurin, a serine-threonine specific phosphatase, is conserved among eukaryotes, and consists of a catalytic and a regulatory subunit, which play a crucial role to maintain the perfect homeostasis of the cell under stress conditions. This protein is activated by calmodulin in response to increases in intracellular calcium and is required to control cell survival under oxidative stress, high temperature and membrane/cell wall perturbation. Fernandes et al., 2005, this issue, pages 216-231, have identified all components of the calcineurin-signaling pathway in the P. brasiliensis transcriptome. According to recent studies, its requirement for growth and virulence-increasing properties in fungal pathogens, such as A. fumigatus, C. neoformans and C. albicans (Odom et al., 1997), make it a potential drug target. Moreover, Odom et al., 1997 proposed calcineurin as the conserved target of two immunosuppressive drugs: cyclosporin A (CsA) and tacrolimus (FK-506), which have been isolated from bacteria and fungi, respectively. In spite of the difference in chemical structures - FK-506 is a macrolide, whereas CsA is a cyclic peptide, they are both hydrophobic and thought to diffuse across the plasma membrane. FK-506 and CsA form complexes with the immunophilins FKBP12 and cyclophilin A (CyPA), respectively. Immunophilins are small, ubiquitous, cytosolic proteins that catalyze cis-trans prolyl isomerization, which is required for protein folding. When FK-506 binds to FKBP12 and CsA binds to CyPA, the inhibition of prolyl-isomerase activity is observed, as is the most exacerbated effect: inhibition of calcineurin signaling through the binding of the drug-immunophilin complexes to the regulatory subunit (Figure 1) (Cardenas et al., 1998). FK-506 and CsA analogs have been described that are specific for fungal calcineurins and also do not cause immunosuppression (Odom et al., 1997). TOR TORs are phosphatidylinositol kinase-related proteins known as key regulators of cell growth in response to nutritional and mitogenic signals and as targets for the immunosuppressive and anti-cancerous drug rapamycin. Rapamycin or sirolimus bind to the immunophilin receptor, FKPB, and the complex rapamycin-Fkpb binds to the Tor protein, blocking signal transduction and arresting cells in G1 (Figure 1) (Brown and Schreiber, 1996). Rapamycin also blocks filamentation in a number of important human and plant pathogens, and its mechanism of action is conserved among eukaryotes. A recent study performed by Steinbach et al. (2004) reports in vitro and in vivo rapamycin activity against A. fumigatus, C. albicans, C. neoformans, and S. cerevisiae (Singh and Heitman, 2004). The rapamycin target TOR protein was found in the P. brasiliensis transcriptome, as well as the downstream components of this pathway (Fernandes et al., 2005, this issue, pages 216-231). The antimicrobial properties of less immunosuppressive analogs of rapamycin hold promise for the development of an effective antifungal therapy (Dickman et al., 2000).
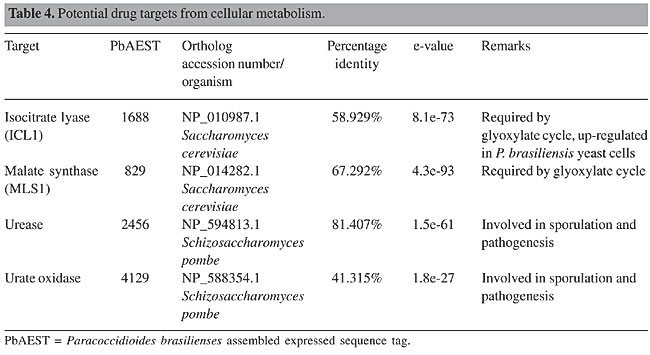
CELL METABOLISM Glyoxylate cycle The glyoxylate cycle is an alternative pathway that helps fungi to obtain energy. Two key enzymes participate in it: isocitrate lyase (Icl1) and malate synthase (Mls1). They are activated when fungus cells are phagocytosed by the macrophage, which appears to be a glucose-limiting environment. The resultant fungal response is an up-regulation of enzymes that use poor carbon sources, including lipids, to synthesize cell wall precursors (Selitrennikoff and Nakata, 2003). These lipids are metabolized into two-carbon units that are brought into the tricarboxylic acid cycle via acetyl-Co-enzyme A. ICL1 cleaves isocitrate, producing glyoxylate and succinate, while MLS1 catalyses the reaction of glyoxylate with acetyl-Co-enzyme A to produce malate. These two reactions in succession bypass the two oxidative decarboxylase steps of the citric cycle (Clemons and Stevens, 2001; Selitrennikoff and Nakata, 2003). Several reports show the importance of the glyoxylate cycle for fungal pathogenicity (Honer et al., 1999; Lorenz and Fink, 2002). Both genes were found in the P. brasiliensis transcriptome (Table 4). These enzymes are not present in human cells and should be assessed for drug design (Lorenz and Fink, 2002; Selitrennikoff and Nakata, 2003).
Urease Urease is a metalloenzyme that catalyses the hydrolysis of urea to ammonia and carbamate. Under physiological conditions, this reaction results in an increase of pH. Many pathogenic fungi have urease activity, among which are C. nerformans, Coccidioides immitis, H. capsulatum, P. brasiliensis (Table 4), Sporothrix sckenchii, and some species of Trichosporon and Aspergillus. The first urease gene cloned from a human pathogenic fungus was that of C. immitis (Yu et al., 1997). In this fungus, the urease gene plays a role in sporulation, pathogenesis and virulence (Cole, 1997). Urease is hypothesized to contribute to alkalinity of the microenvironment in which the fungus grows, mainly due to the release of ammonia and ammonium ions. However, it is evident that C. immitis urease activity is not responsible for the total amount of ammonia secreted during in vitro growth of the pathogen (Mirbod et al., 2002). Being a virulence factor in pathogens, including fungi and bacteria, and absent in humans, urease is an important enzyme involved in the colonization of the host, and it may serve as a potential drug target. Urate oxidase (uricase) Urate oxidase or uricase is an enzyme of the purine degradation pathway that was found in the P. brasiliensis transcriptome (Table 4). It is responsible for the conversion of uric acid into allantoin and hydrogen peroxide (Nahm and Marzluf, 1987). Functional uricase is absent in higher primates, which excrete uric acid as the end product of purine degradation (Elion et al., 1968; Friedman et al., 1985). Since uricase is a powerful scavenger of free radicals, it plays an important role in protecting pathogens during macrophage ingestion (Ames et al., 1981; Whiteman and Halliwell, 1996), and therefore its blockage may lead to their impaired growth. ESSENTIAL GENES Roemer et al. (2003) developed a very efficient strategy to identify essential genes in the human pathogen C. albicans. The technique called GRACETM (gene replacement and conditional expression) is based on deletion of the first allele (C. albicans is a diploid organism) by PCR-generated cassettes containing a selectable marker, and a tetracycline-repressed promoter linked to a second selectable marker replaces the second allele. By this approach, Roemer et al., 2003 studied 1152 genes, but only 61% (567) were confirmed experimentally to be essential in C. albicans. Most of the genes are required for cellular processes such as cell growth and division, as well as DNA synthesis. The main goal of the present study is that by this technique it is possible to identify and validate the target in vitro and in vivo, in C. albicans, an opportunist diploid pathogen with no obvious sexual cycle, which impairs classic genetic analysis. Based on the similarities with our biological model of interest, P. brasiliensis, the GRACETM strategy is a very promising way to screen for essential genes and further develop novel therapies against paracoccidioidomycosis. In our analysis, we have decided to search for the C. albicans essential genes in the P. brasiliensis transcriptome; we identified 15 genes, such as alg7 (UDP-N-acetylglucosamine-1-phosphate transferase) and sec14 (phosphatidylinositol/phosphatidylcholine transferase). Though they are present in P. brasiliensis, further studies such as knockout or GRACE experiments, may confirm their true importance in this organism. CONCLUDING REMARKS We have explored different possible targets for the development of alternative drug therapies against P. brasiliensis. Some of the potential targets discussed here, which are fungus-specific and have been dissected as promising targets for the majority of fungal pathogens, were identified in our P. brasiliensis transcriptome analyses. Targets present in both host and pathogen should not be discarded due to the possibility to explore their differences, as is the case for Top1. Since current therapy is aimed at targets related to plasma membrane biosynthesis of both fungi and mammals, the respective drugs appear to be specific for the fungal enzymes. Bearing this in mind, the refinement of current antifungals is a great option to develop new specific therapies. One source of potential targets that was recently proposed by Roemer et al. (2003) is the identification of essential genes. Our comparative study was made possible by the P. brasiliensis transcriptome-sequencing project, which has enabled us to compare and validate targets described in other pathogenic organisms with the expressed sequence tags found in our database. Together with the great advances in understanding fungal pathogenesis, the development of methods for screening in combinatorial chemistry libraries and improved molecular modeling computational programs, the molecular revolution holds new promises and perspectives in the newly born field of antifungal design. ACKNOWLEDGMENTS Research supported by MCT/CNPq, CNPq, CAPES, FUB, and UFG. We are thankful to Hugo Costa Paes for English revision of this text. REFERENCES Albuquerque, P., Kyaw, C.M., Saldanha, R.R., Brigido, M.M., Felipe, M.S.S. and Silva-Pereira, I. (2004). Pbhyd1 and Pbhyd2: two mycelium-specific hydrophobin genes from the dimorphic fungus Paracoccidioides brasiliensis. Fungal Genet. Biol. 41: 510-520. Ames, B.N., Cathcart, R., Schwiers, E. and Hochstein, P. (1981). Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc. Natl. Acad. Sci. USA 78: 6858-6862. Andriole, V.T. (2000). Current and future antifungal therapy: new targets for antifungal therapy. Int. J. Antimicrob. Agents 16: 317-321. Barrett, D. (2002). From natural products to clinically useful antifungals. Biochim. Biophys. Acta 1587: 224-233. Beauvais, A. and Latgé, J.P. (2001). Membrane and cell wall targets in Aspergillus fumigatus. Drug Resist. Update 4: 38-49. Beauvais, A., Bruneau, J.M., Mol, P.C., Buitrago, M.J., Legrand, R. and Latge, J.P. (2001). Glucan synthase complex of Aspergillus fumigatus. J. Bacteriol. 183: 2273-2279. Bernard, M. and Latgé, J.P. (2001). Aspergillus fumigatus cell wall: composition and biosynthesis. Med. Mycol. 39: 9-17. Bowman, B.J. and Bowman, E.J. (2002). Mutations in subunit c of the vacuolar ATPase confer resistance to bafilomycin and identify a conserved antibiotic binding site. J. Biol. Chem. 277: 3965-3972. Brown, E.J. and Schreiber, S.L. (1996). A signaling pathway to translational control. Cell 86: 517-520. Brummer, E., Sun, S.H., Harrison, J.L., Perlman, A.M., Philpott, D.E. and Stevens, D.A. (1990). Ultrastructure of phagocytosed Paracoccidioides brasiliensis in nonactivated or activated macrophages. Infect. Immun. 58: 2628-2636. Bulawa, C.E., Slater, M., Cabib, E., Au-Young, J., Sburlati, A., Adair Jr., W.L. and Robbins, P.W. (1986). The Saccharomyces cerevisiae structural gene for chitin synthase is not required for chitin in vivo. Cell 46: 213-225. Burghoorn, H.P., Soteropoulos, P., Paderu, P., Kashiwazaki, R. and Perlin, D.S. (2002). Molecular evaluation of the plasma membrane proton pump from Aspergillus fumigatus. Antimicrob. Agents Chemother. 46: 615-624. Cabib, E. (1991). Differential inhibition of chitin synthases 1 and 2 from Saccharomyces cerevisiae by polyoxin D and nikkomycins. Antimicrob. Agents Chemother. 35: 170-173. Calonge, T.M., Nakano, K., Arellano, M., Arai, R., Katayama, S., Toda, T., Mabuchi, I. and Perez, P. (2000). Schizosaccharomyces pombe rho2p GTPase regulates cell wall alpha-glucan biosynthesis through the protein kinase pck2p. Mol. Biol. Cell. 11: 4393-4401. Cardenas, M.E., Sanfridson, A., Cutler, N.S. and Heitman, J. (1998). Signal-transduction cascades as targets for therapeutic intervention by natural products. TIBTECH 16: 427-433. Clemons, K.V. and Stevens, D.A. (2001). Overview of host defense mechanisms in systemic mycoses and the basis for immunotherapy. Semin. Respir. Infect. 16: 60-66. Cole, G.T. (1997). Ammonia production by Coccidioides immitis and its possible significance to the host-fungus interplay. In: Host-Fungus Interplay (van den Bossche, H., Stevens, D.A. and Odds, F.C., eds.). National Foundation for Infectious Disease, Bethesda, MD, USA, p. 247-263. Costa, A.A., Gómez, F.J., Pereira, M., Felipe, M.S.S., Jesuíno, R.S.A., Deep Jr., G.S. and Soares, C.M.A. (2002). Characterization of a gene which encodes a mannosyltransferase homolog of Paracoccidioides brasiliensis. Microbes Infect. 4: 1027-1034. D’Arpa, P. and Liu, L.F. (1989). Topoisomerase-targeting antitumor drugs. Biochim. Biophys. Acta 989: 163-177. Dávila, T., San-Blas, G. and San-Blas, F. (1986). Effect of papulacandin B on glucan synthesis in Paracoccidioides brasiliensis. J. Med. Vet. Mycol. 24: 193-202. Dickman, D.A., Ding, H., Li, Q., Nilius, A.M., Balli, D.J., Ballaron, S.J., Trevillyan, J.M., Smith, M.L., Seif, L.S., Kim, K., Sarthy, A., Goldman, R.C., Plattner, J.J. and Bennani, Y.L. (2000). Antifungal rapamycin analogues with reduced immunosuppressive activity. Bioorg. Med. Chem. Lett. 10: 1405-1408. Douglas, C.M. (2001). Fungal b(1,3)-D-glucan synthesis. Med. Mycol. 39: 55-66. Eaton, K.A., Brooks, C.L., Morgan, D.R. and Krakowka, S. (1991). Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect. Immun. 59: 2470-2475. Elion, G.B., Yu, T.F., Gutman, A.B. and Hitchings, G.H. (1968). Renal clearance of oxipurinol, the chief metabolite of allopurinol. Am. J. Med. 45: 69-77. Ernst, J.F. and Prill, S.K. (2001). O-glycosylation. Med. Mycol. 39 (Suppl. 1): 67-74. Felipe, M.S., Andrade, R.V., Arraes, F.B., Nicola, A.M., Maranhao, A.Q., Torres, F.A., Silva-Pereira, I., Pocas-Fonseca, M.J., Campos, E.G., Moraes, L.M., Andrade, P.A., Tavares, A.H., Silva, S.S., Kyaw, C.M., Souza, D.P., Network P., Pereira, M., Jesuino, R.S., Andrade, E.V., Parente, J.A., Oliveira, G.S., Barbosa, M.S., Martins, N.F., Fachin, A.L., Cardoso, R.S., Passos, G.A., Almeida, N.F., Walter, M.E., Soares, C.M., Carvalho, M.J. and Brigido, M.M. (2005). Transcriptional profiles of the human pathogenic fungus Paracoccidioides brasiliensis in mycelium and yeast cells. J. Biol. Chem. 280: 24706-24714. Fernandes, L., Araújo, M.A.M., Amaral, A., Reis, V.C.B., Martins, N.F. and Felipe, M.S. (2005). Cell signaling pathways in Paracoccidioides brasiliensis - inferred from comparisons with other fungi. Genet. Mol. Res. 4: 216-231. Fostel, J.M. and Montgomery, D.A. (1995). Identification of the aminocatechol A-3253 as an in vitro poison of DNA topoisomerase I from Candida albicans. Antimicrob. Agents Chemother. 39: 586-592. Fostel, J.M., Montgomery, D.A. and Shen, L.L. (1992). Characterization of DNA topoisomerase I from Candida albicans as a target for drug discovery. Antimicrob. Agents Chemother. 36: 2131-2138. Friedman, T.B., Polanco, G.E., Appold, J.C. and Mayle, J.E. (1985). On the loss of uricolytic activity during primate evolution-I. Silencing of urate oxidase in a hominoid ancestor. Comp. Biochem. Physiol. B 81: 653-659. Fukui, H., Koike, T., Saheki, A., Sonoke, S. and Seki, J. (2003). A novel delivery system for amphotericin B with lipid nano-sphere (LNS). Int. J. Pharm. 265: 37-45. Gaughran, J.P., Lai, M.H., Kirsch, D.R. and Silverman, S. (1994). Nikkomycin Z is a specific inhibitor of Saccharomyces cerevisiae chitin synthase isoenzyme Chs3 in vitro and in vivo. J. Bacteriol. 176: 5857-5860. Georgopapadakou, N.H. and Walsh, T.J. (1996). Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob. Agents Chemother. 40: 279-291. Goldman, R.C., Sullivan, P.A., Zakula, D. and Capobianco, J.O. (1995). Kinetics of beta-1,3 glucan interaction at the donor and acceptor sites of the fungal glucosyltransferase encoded by the BGL2 gene. Eur. J. Biochem. 227: 372-378. Gooday, G.W. (1995). The dynamics of hyphal growth. Mycol. Res. 99: 385-394. Gozalbo, D., Patricia, R., Villamón, E. and María, L.G. (2004). Candida and candidiase: The cell wall as a potential molecular target for antifungal therapy. Curr. Drug Target Infect. Disord. 4: 117-135. Hanson, L.H. and Stevens, D.A. (1989). Evaluation of cilofungin, a lipopeptide antifungal agent, in vitro against fungi isolated from clinical specimens. Antimicrob. Agents Chemother. 33: 1391-1392. Hearn, V.M., Escott, G.M., Glyn, E., Evans, V. and Adams, D.J. (1998). Complex chitinolytic system of Aspergillus fumigatus. Microbios 93: 85-104. Heung, L.J., Luberto, C., Plowden, A., Hannun, Y.A. and Del Poeta, M. (2004). The sphingolipid pathway regulates Pkc1 through the formation of diacylglycerol in Cryptococcus neoformans. J. Biol. Chem. 279: 21144-21153. Hogan, L. and Klein, B. (1994). Altered expression of surface alpha-1,3-glucan in genetically related strains of Blastomyces dermatitidis that differ in virulence. Infect. Immun. 62: 3543-3546. Honer, Z.B.K., Miczak, A., Swenson, D.L. and Russell, D.G. (1999). Characterization of activity and expression of isocitrate lyase in Mycobacterium avium and Mycobacterium tuberculosis. J. Bacteriol. 181: 7161-7167. Horiuchi, H. and Takagi, M. (1999). Chitin synthase genes of Aspergillus species. In: Aspergillus fumigatus Contributions in Microbiology (Brakhage, A.A., Jahn, B. and Schmidt, A., eds.). Karger, Basel, Switzerland, pp.193-204. Jiang, W., Gerhold, D., Kmiec, E.B., Hauser, M., Becker, J.M. and Koltin, Y. (1997). The topoisomerase I gene from Candida albicans. Microbiology 143: 377-386. Kanetsuna, F., Carbonell, L.M., Azuma, I. and Yamamura, Y. (1972). Biochemical studies on the thermal dimorphism of Paracoccidioides brasiliensis. J. Bacteriol. 110: 208-218. Kelly, R., Register, E., Hsu, M.J., Kurtz, M. and Nielsen, J. (1996). Isolation of a gene involved in 1,3-beta-glucan synthesis in Aspergillus nidulans and purification of the corresponding protein. J. Bacteriol. 178: 4381-4391. Klepser, M.E. (2003). Future candidates in the search for new antifungal agents. Curr. Treat. Opt. Infect. Dis. 5: 489-494. Klimpel, K.R. and Goldman, W.E. (1988). Cell walls from avirulent variants of Histoplasma capsulatum lack alpha-(1,3)-glucan. Infect. Immun. 56: 2997-3000. Klis, F.M., De Groot, P. and Hellingwerf, K. (2001). Molecular organization of the cell wall of Candida albicans. Med. Mycol. 39 (Suppl 1): 1-8. Kohl, N.E., Omer, C.A., Conner, M.W., Anthony, N.J., Davide, J.P., de Solms, S.J., Giuliani, E.A., Gomez, R.P., Graham, S.L. and Hamilton, K. (1995). Inhibition of farnesyltransferase induces regression of mammary and salivary carcinomas in ras transgenic mice. Nat. Med. 1: 792-797. Kondoh, O., Tachibana, Y., Ohya, Y., Arisawa, M. and Watanabe, T. (1997). Cloning of the RHO1 gene from Candida albicans and its regulation of b-1,3-glucan synthesis. J. Bacteriol. 179: 7734-7741. Kontoyiannis, D.P. and Lewis, R.E. (2002). Antifungal drug resistance of pathogenic fungi. Lancet 359: 1135-1144. Lee, M.C.S., Hamamoto, S. and Schekman, R. (2002). Ceramide biosynthesis is required for the formation of the oligomeric H+ATPase Pma1p in the yeast endoplasmic reticulum. J. Biol. Chem. 277: 22395-22401. Lodge, J.K., Jackson-Machelski, E., Toffaletti, D.L., Perfect, J.R. and Gordon, J.I. (1994). Targeted gene replacement demonstrates that myristoyl-CoA:protein N-myristoyltransferase is essential for viability of Cryptococcus neoformans. Proc. Natl. Acad. Sci. USA 91: 12008-12012. Lorenz, M.C. and Fink, G.R. (2001). The glyoxylate cycle is required for fungal virulence. Nature 412: 83-86. Lorenz, M.C. and Fink, G.R. (2002). Life and death in a macrophage: role of the glyoxylate cycle in virulence. Eukaryot. Cell 1: 657-662. Luberto, C., Toffaletti, D.L., Wills, E.A., Tucker, S.C., Casadevall, A., Perfect, J.R., Hannun, Y.A. and Del Poeta, M. (2001). Roles of inositol-phosphoryl ceramide synthase 1 (IPC1) in pathogenesis of C. neoformans. Genes Dev. 15: 201-212. Luo, H., Morsomme, P. and Boutry, M. (1999). The two major types of plant plasma membrane H+-ATPases show different enzymatic properties and confer differential pH sensitivity of yeast growth. Plant Physiol. 119: 627-634. Matthews, R.C., Maresca, B., Burnie, J.P., Cardona, A., Carratu, L., Conti, S., Deepe, G.S., Florez, A.M., Franceschelli, S., Garcia, E., Gargano, L.S., Kobayashi, G.S., McEwen, J.G., Ortiz, B.L., Oviedo, A.M., Polonelli, L., Ponton, J., Restrepos, A. and Storlazzi, A. (1998). Stress proteins in fungal diseases. Med. Mycol. 36: 45-51. McGeady, P., Logan, D.A. and Wansley, D.L. (2002). A protein-farnesyl transferase inhibitor interferes with the serum-induced conversion of Candida albicans from a cellular yeast form to a filamentous form. FEMS Microbiol. Lett. 213: 41-44. Mellor, K.J., Nicholas, R.O. and Adams, D.J. (1994). Purification and characterization of chitinase from Candida albicans. FEMS Microbiol. Lett. 119: 111-118. Mirbod, F., Schaller, R.A. and Cole, G.T. (2002). Purification and characterization of urease isolated from the pathogenic fungus Coccidioides immitis. Med. Mycol. 40: 35-44. Mouyna, I., Sarfati, J., Recco, P., Fontaine, T., Henrissat, B. and Latge, J.P. (2002). Molecular characterization of a cell wall-associated b(1-3)endoglucanase of Aspergillus fumigatus. Med. Mycol. 40: 455-464. Nagarajan, S.R., Devadas, B., Zupec, M.E., Freeman, S.K., Brown, D.L., Lu, H.F., Mehta, P.P., Kishore, N.S., McWherter, C.A., Getman, D.P., Gordon, J.I. and Sikorski, J.A. (1997). Conformationally constrained [p-(omega-aminoalkyl)phenacetyl]-L-seryl-L-lysyl dipeptide amides as potent peptidomimetic inhibitors of Candida albicans and human myristoyl-CoA:protein N-myristoyl transferase. J. Med. Chem. 40: 1422-1438. Nahm, B.H. and Marzluf, G.A. (1987). Induction and de novo synthesis of uricase, a nitrogen-regulated enzyme in Neurospora crassa. J. Bacteriol. 169: 1943-1948. Nakai, T., Uno, J., Ikeda, F., Tawara, S., Nishimura, K. and Miyaji, M. (2003). In vitro antifungal activity of Micafungin (FK463) against dimorphic fungi: comparison of yeast-like and mycelial forms. Antimicrob. Agents Chemother. 47: 1376-1381. Niño-Vega, G.A., Buurman, E.T., Gooday, G.W., San-Blas, G. and Gow, N.A.R. (1998). Molecular cloning and sequencing of a chitin synthase gene (CHS2) of Paracoccidioides brasiliensis. Yeast 14: 181-187. Niño-Vega, G.A., Munro, C.A., San-Blas, G., Gooday, G.W. and Gow, N.A.R. (2000). Differential expression of chitin synthase genes during temperature-induced dimorphic transition in Paracoccidioides brasiliensis. Med. Mycol. 38: 31-39. Obeid, L.M., Okamoto, Y. and Mao, C. (2002). Yeast sphingolipids: metabolism and biology. Biochim. Biophys. Acta. 1585: 163-171. Odds, F.C., Brown, A.J.P. and Gow, N.A.R. (2003). Antifungal agents: mechanisms of action. Trends Microbiol. 11: 272-279. Odom, A., del Poeta, M., Perfect, J. and Heitman, J. (1997). The immunosuppressant FK506 and its nonimmunosuppressive analog L-685,818 are toxic to Cryptococcus neoformans by inhibition of a common target protein. Antimicrob. Agents Chemother. 41: 156-161. Onishi, J., Meinz, M., Thompson, J., Curotto, J., Dreikorn, S., Rosenbach, M., Douglas, C., Abruzzo, G., Flattery, A., Kong, L., Cabello, A., Vicente, F., Pelaez, F., Diez, M.T., Martin, I., Bills, G., Giacobbe, R., Dombrowski, A., Schwartz, R., Morris, S., Harris, G., Tsipouras, A., Wilson, K. and Kurtz, M.B. (2000). Discovery of novel antifungal (1,3)-beta-D-glucan synthase inhibitors. Antimicrob. Agents Chemother. 44: 368-377. Onyewu, C., Blankenship, J.R., Del Poeta, M. and Heitman, J. (2003). Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata and Candida krusei. Antimicrob. Agents Chemother 47: 956-964. Parang, K., Knaus, E.E., Wiebe, L.I., Sardari, S., Daneshtalab, M. and Csizmadia, F. (1996). Synthesis and antifungal activities of myristic acid analogs. Arch. Pharm. 329: 475-482. Pereira, M., Felipe, M.S.S., Brígido, M.M., Soares, C.M.A. and Azevedo, M.O. (2000). Molecular cloning and characterization of a glucan synthase gene from the human pathogenic fungus Paracoccidioides brasiliensis. Yeast 16: 451-462. Piper, P.W. (2001). The Hsp90 chaperone as a promising drug target. Curr. Opin. Investig. Drugs 2: 1606-1610. Pizzirusso, M. and Chang, A. (2004). Ubiquitin-mediated targeting of a mutant plasma membrane ATPase, Pma1-7, to the endosomal/vacuolar system in yeast. Mol. Biol. Cell. 15: 2401-2409. Popolo, L. and Vai, M. (1999). The Gas1 glycoprotein, a putative wall polymer cross-linker. Biochim. Biophys. Acta 1426: 385-400. Rocque, W.J., McWherter, C.A., Wood, D.C. and Gordon, J.I. (1993). A comparative analysis of the kinetic mechanism and peptide substrate specificity of human and Saccharomyces cerevisiae myristoyl-CoA:protein N-myristoyltransferase. J. Biol. Chem. 268: 9964-9971. Rodrigues-Peña, J.M., Cid, V.J., Arroyo, J. and Nombela, C. (2000). A novel family of cell wall-related proteins regulated differently during the yeast life cycle. Mol. Cell. Biol. 20: 3245-3255. Roemer, T., Jiang, B., Davison, J., Ketela, T., Veillette, K., Breton, A., Tandia, F., Linteau, A., Sillaots, S., Marta, C., Martel, N., Veronneau, S., Lemieux, S., Kauffman, S., Becker, J., Storms, R., Boone, C. and Bussey, H. (2003). Large-scale essential gene identification in Candida albicans and applications to antifungal drug discovery. Mol. Microbiol. 50: 167-181. Ruiz-Herrera, J. (1992). Fungal Cell Wall: Structure, Synthesis and Assembly. CRC Press, Boca Raton, FL, USA. Ruiz-Herrera, J. and San Blas, G. (2003). Chitin synthesis as target for antifungal drugs. Curr. Drug Targets Infect. Disord. 3: 77-91. San-Blas, G. (1979). Biosynthesis of glucans by subcellular fractions of Paracoccidioides brasiliensis. Exp. Mycol. 3: 249-258. San-Blas, G., San-Blas, F. and Serrano, L.E. (1977). Host parasite relationships in the yeast like form of Paracoccidioides brasiliensis strain IVIC Pb9. Infect. Immun. 15: 343-346. Santos, M.O., Pereira, M., Felipe, M.S.S., Jesuino, R.S.A., Ulhoa, C.J., Soares, R.B.A. and Soares, C.M.A. (2004). Molecular cloning and characterization of a cDNA encoding the N-acetyl-b-D-glucosaminidase homologue of Paracoccidioides brasiliensis. Med. Mycol. 42: 247-253. Schafer, W.R. and Rine, J. (1992). Protein prenylation: genes, enzymes, targets, and functions. Annu. Rev. Genet. 26: 209-237. Selitrennikoff, C.P. and Nakata, M. (2003). New cell wall targets for antifungal drugs. Curr. Opin. Investig. Drugs 4: 200-205. Silverman, S.J., Sburlati, A., Slater, M.L. and Cabib, E. (1988). Chitin sunthase 2 is essential for septum formation and cell division in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 85: 4735-4739. Singh, N. and Heitman, J. (2004). Antifungal attributes of immunosuppressive agents: new paradigms in management and elucidating the pathophysiologic basis of opportunistic mycoses in organ transplant recipients. Transplantation 77: 795-800. Smith, W.L. and Edlind, T.D. (2002). Histone deacetylase inhibitors enhance Candida albicans sensitivity to azoles and related antifungals: correlation with reduction in CDR and ERG upregulation. Antimicrob. Agents Chemother. 46: 3532-3539. Sobue, S. and Sekiguchi, K. (2004). Difference in percutaneous absorption and intracutaneous distribution in guinea pigs among topical antifungal drugs (tioconazole solution, tioconazole cream, miconazole nitrate solution and bifonazole solution). Biol. Pharm. Bull. 27: 1428-1432. Steinbach, W.J., Schell, W.A., Blankenship, J.R., Onyewu, C., Heitman, J. and Perfect, J.R. (2004). In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus. Antimicrob. Agents Chemother. 48: 1664-1669. Stewart, L., Ireton, G.C. and Champoux, J.J. (1996). The domain arrangement of human topoisomerase I. J. Biol. Chem. 271: 7602-7608. Stewart, L., Redinbo, M.R., Qiu, X., Hol, W.G. and Champoux, J.J. (1998). A model for the mechanism of human topoisomerase I. Science 279: 1534-1541. Tanaka, K., Nambu, H., Katoh, Y., Kai, M. and Hidaka, Y. (1999). Molecular cloning of homologs of RAS and RHO1 genes from Cryptococcus neoformans. Yeast 15: 1133-1139. Theis, T. and Stahl, U. (2004). Antifungal proteins: targets, mechanisms and prospective applications. Cell Mol. Life Sci. 61: 437-455. Thevissen, K., Warnecke, D.C., François, I.E.J.A., Leipelt, M., Heinz, E., Ott, C., Zähringedr, U., Thomma, B.P.H.J., Ferket, K.K.A. and Cammue, B.P.A. (2004). Defensins from insects and plants interact with fungal glucosylceramides. J. Biol. Chem. 279: 3900-3905. Tkacz, J.S. and DiDomenico, B. (2001). Antifungals: what’s in the pipeline. Curr. Opin. Microbiol. 4: 540-545. Vallim, M.A., Fernandes, L. and Alspaugh, J.A. (2004). The RAM1 gene encoding a proteinfarnesyltransferase b-subunit homologue is essential in Cryptococcus neoformans. Microbiology 150: 1925-1935. Wang, Q. and Chang, A. (2002). Sphingolipid base synthesis is required for oligomerization and cell surface stability of the yeast plasma membrane ATPase, Pma1. Proc. Natl. Acad. Sci. USA 99: 12853-12858. Wessels, J.G.H. (1988). A steady-state model for apical wall growth in fungi. Acta Bot. Neerl. 37: 3-16. Whiteman, M. and Halliwell, B. (1996). Protection against peroxynitrite-dependent tyrosine nitration and alpha 1-antiproteinase inactivation by ascorbic acid. A comparison with other biological antioxidants. Free Radic. Res. 25: 275-283. Wills, E.A., Redinbo, M.R., Perfect, J.R. and Del Poeta, M. (2000). New potential targets for antifungal development. Emerg. Ther. Targets 4: 1-42. Young, L.Y., Hull, C.M. and Heitman, J. (2003). Disruption of ergosterol biosynthesis confers resistance to amphotericin B in Candida lusitaniae. Antimicrob. Agents Chemother. 49: 2717-2724. Yu, J.J., Smithson, S.L., Thomas, P.W., Kirkland, T.N. and Cole, G.T. (1997). Isolation and characterization of the urease gene (URE) from the pathogenic fungus Coccidioides immitis. Gene 198: 387-391. Zhong, W., Jeffries, M.W. and Georgopapadakou, N.H. (2000). Inhibition of inositol phosphorylceramide synthase by Aureobasidin A in Candida and Aspergillus species. Antimicrob. Agents Chemother. 44: 651-653. |
|