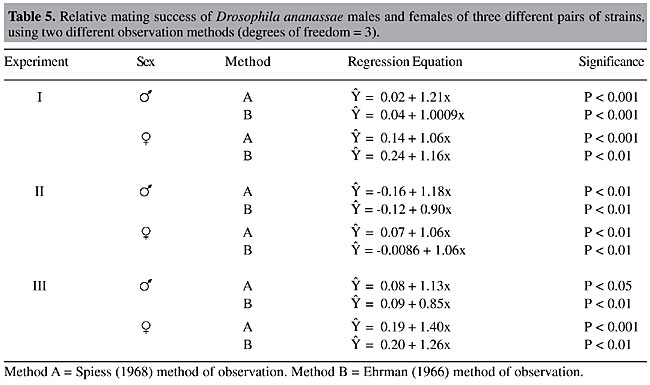
ABSTRACT. Frequency-dependent mating success was tested for three pairs of wild-type and mutant strains of Drosophila ananassae, MY and yellow body color (y), PN and claret eye color (ca), and TIR and cut wing (ct). The two strains of each pair were chosen for their approximately equal mating propensities. Multiple-choice experiments, using different experimental procedures, were employed. The tests were carried out by direct observation in Elens-Wattiaux mating chambers with five different sex ratios (4:16, 8:12, 10:10, 12:8, and 16:4). There was no assortative mating and sexual isolation between the strains, based on 2 x 2 contingency c2 analysis and isolation estimate values. One-sided rare male mating advantages were found in two experiments, one for ca males and the other for wild-type males (TIR). However, no advantage was found for rare males in the experiment with MY and y flies. Mating disadvantages for rare females were found for sex-linked mutants (y and ct). Two different observational methods (removal or direct observation of mating pairs) imparted no overall significant effects on the outcome of the frequency-dependent mating tests. Key words: Drosophila ananassae, Multiple-choice experiments, Minority-mating success, Mutant flies, Wild-type flies INTRODUCTION At least a third of all loci are polymorphic. Various models have been postulated to explain the stability of this genetic polymorphism found in natural populations, the most commonly cited of which is heterozygote superiority. Another popular model is frequency-dependent selection, which favors the rare type for different fitness traits, including sexual activity. The most argued component of this frequency-dependent selection is minority-mating advantage, a form of frequency-dependent sexual selection. Minority-mating advantage means that a rare competing genotype is favored for mating, regardless of its type, until equilibrium is reached in a population. This model is expected to maintain genetic variation without heterosis and its accompanying genetic load, as at equilibrium frequencies the fitnesses of different types are equal (Anderson, 1969; Lewontin, 1974). Though the rare type will be favored and increase in frequency, it will never become fixed through the generations, causing extinction of the other strain, as once it is common, its advantage will be lost (Adams and Duncan, 1979). Also, it is suggested that rare male mating advantage promotes outbreeding, because an occasional male from another population is preferred for mating (Dal Molin, 1979; Grant et al., 1980). However, Ball et al. (2000) did not confirm this idea. They reported that the rare male effect had little impact on the fitness advantage of the immigrant allele in a study of the genetic contribution of single male immigrants to small, inbred populations of Drosophila melanogaster. Minority-mating advantage was discovered independently by Petit (1951) and Ehrman (1966). Since then, rigorous studies have been carried out by several authors, involving both empirical and statistical analyses on this phenomenon (see Knoppien, 1985a; Partridge, 1988; Singh and Sisodia, 2000). Arguments and counterarguments regarding its existence and mechanisms have made this hypothesis a most intriguing one. Despite a moderately long research history of half-a-century, the idea of minority-mating advantage has still to find a secure place in genetics test books. Evidence, found both for and against this type of frequency-dependent sexual selection, however, has kept this question open, emphasizing the need for more work on this phenomenon. Minority-mating advantage has been reported in 12 species of Drosophila and also in some non-drosophilid flies, as well as in a few vertebrates (Knoppien, 1985a; Singh and Sisodia, 2000). Intraspecifically, the rare male effect has been tested using different wild-type strains, mutants, strains having different chromosome arrangements, allozyme variants, behavioral characters, the same strains reared at different temperatures (Knoppien, 1985a; Singh and Sisodia, 2000), and by isolating males and females during different developmental stages (Ehrman and Kim, 1995). Singh and Chatterjee (1989) reported a rare male mating advantage in D. ananassae using se and cd mutants and wild-type strains. Singh and Sisodia (1997) found evidence for a rare male effect in D. bipectinata. Singh and Som (2001) and Som and Singh (2004) reported a one-sided rare male mating advantage in a study made with two different karyotypic strains of D. ananassae; however, they found no evidence for minority-mating success in wild-type strains of the same species (Som and Singh, 2002). We carried out experiments to study rare type mating advantage using mutant and wild-type flies of D. ananassae. D. ananassae belongs to the ananassae species complex of the ananassae subgroup of the melanogaster species group. Although it is cosmopolitan in distribution, it is largely circumtropical and it is commonly found in India. This species is unique in the genus Drosophila due to certain peculiarities in its genetic behavior, especially due to its spontaneous male meiotic recombination at an appreciable frequency, which occurs at a very low rate in other species, including D. melanogaster, and for its high mutability (see, Singh, 1996, 2000). Also, like typical cosmopolitan species, D. ananassae males exhibit a high sexual drive, and females have a relatively high discriminating capacity (Spieth, 1966). All these characteristics make D. ananassae a useful model to study sexual behavior. Multiple-choice experiments, where two types of females are confined with two types of males, give a close approach to the natural condition. They permit observations of all four combinations of matings between two types of male and female flies. Also, in this type of choice experiment, both types of females get an opportunity to choose simultaneously. In multiple-choice trials, interactions between the female types can also take place, which is not possible in other choice situations, e.g., female choice, where one type of female is kept with two types of males (Peterson and Merrell, 1983). In most of the literature non-random mating is attributed to female discrimination, while male choice has been ignored. Though males are by and large considered to indiscriminately court females, Noor (1996) suggests that mating discrimination by females and males is approximately equally frequent in Drosophila. Thus, multiple-choice experiments might be more informative than female choice for studying mate preference and a possible consequent rare type advantage. MATERIAL AND METHODS Three mutant strains of D. ananassae, yellow body color (y66), claret eye (ca) and cut wing (ct5) were studied for rare male effect against three wild-type strains, MY (Mysore), PN (Pune) and TIR (Tirupati), respectively. Each of these wild-type strains was chosen based on an initial mating propensity test, in which each strain was found approximately equal to the respective mutant strain. This precaution was taken because differences in mating propensities strongly affect the multiple-choice test, which can lead to misinterpretation of discrimination (Casares et al., 1998). Also, if one type of male is sexually more active, a one-sided rare male mating advantage favors the more vigorous type (Bryant et al., 1980). Description of the mutant strains y66 is a sex-linked recessive mutation, having a yellow body color (Tobari, 1993), with yellow wings and bristles, due to less than normal pigmentation of the cuticle. ca is a recessive mutation on chromosome two, with brownish eyes that darken with age (Tobari, 1993). It has homology with ca of D. melanogaster, where it was found that the claret mutation causes a reduction in the levels of both pteridines (red pigments) and ommochromes (brown pigments) (Sequeira et al., 1989). ct5 is a sex-linked recessive mutation, with pointed wings due to marginal excisions (Tobari, 1993). In D. melanogaster, most alleles of the cut locus are pleiotropic, exhibiting various combinations of aberrations (Johnson and Judd, 1979). In our strain of D. ananassae we found failure of wing expansion and sometimes a small blister associated with the cut wing. We only used flies with fully expanded and blisterless wings. The wild-type mass cultures PN and TIR were established from flies collected in 1999 from Pune and Tirupati, India, respectively, and MY from Mysore, India, in 2000. In order to understand the effects of ‘rarity’ on a single locus, we tried to ‘randomize’ the differences between the genetic backgrounds of mutant and wild-type flies by crossing the flies of a mutant strain with the flies of a wild-type strain in reciprocal crosses. Males and females, generated from both crosses, were mixed and were maintained for several generations. Mutant and wild-type lines were established by pair mating for the yellow and cut mutations. In order to establish the wild-type line for the autosomal recessive mutant (claret), the vials in which claret flies appeared after pair mating were rejected, and only those having wild phenotypes were considered. Male and female parents of each of this type of vial were stored separately in food vials. Stored sperm in females were exhausted totally by changing the food vials every two or three days. Then females were backcrossed to test for homozygosity. Males were also tested for homozygosity. The wild-type line was established by only taking flies generated from parents found homozygous for wild type. In this way we sought to achieve randomization at the other loci, except in the vicinity of the mutant locus. However, very closely linked loci may or may not be randomized in this way. Within a few hours after eclosion, both virgin females and males were isolated under light ether anesthesia. Flies of each sex were stored in separate food vials (7.5 cm in length and 2.2 cm in diameter) in batches of 15 to avoid bias in the outcome of the rare male test due to a density effect (Knoppien, 1985b; Knoppien, 1987). The flies were aged for seven days. One day before each experiment, 20 females and 20 males were stored separately in fresh food vials at the ratios to be tested (five ratios, 4:16, 8:12, 10:10, 12:8, 16:4) using a very low dose of ether. Care was taken to avoid sampling errors (Markow, 1980). Due to the distinguishable phenotypes, there was no need to mark the flies in order to differentiate between the two types of flies. Ratios of males and females were varied simultaneously. Six replicates were carried out for each ratio. Mating success was observed directly by introducing flies into a Elens-Wattiaux (Elens and Wattiaux, 1964) mating chamber (10.5 cm in diameter), without etherization. First, females were introduced and then males. The general sex ratio was 1:1. Knoppien (1985a) has shown that the magnitude of the rare male mating advantage depends on the experimental approach. We sought to determine whether the method of observation affects the results of minority effect experiments. The mated flies were counted by the following two methods (see, Knoppien, 1985a):
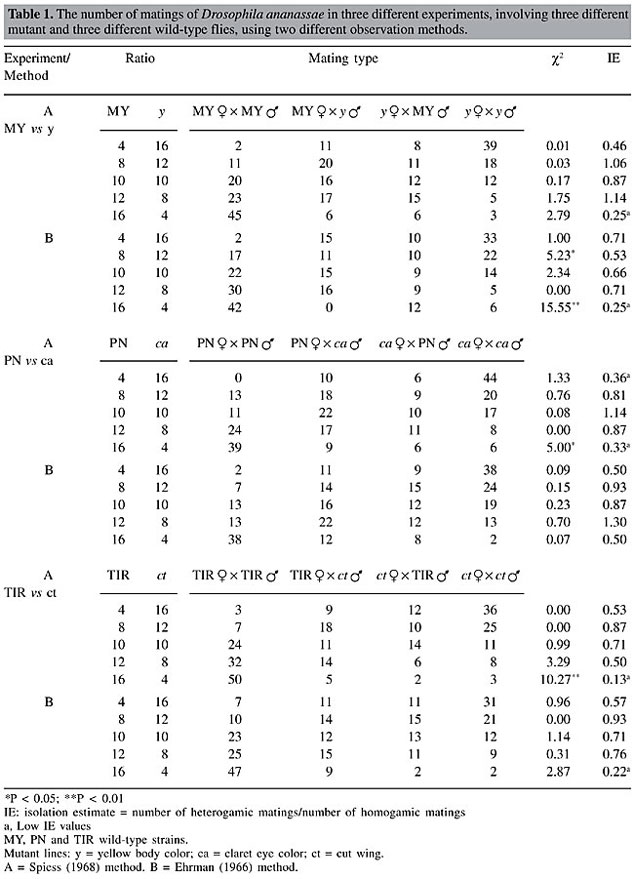
For each replicate, the first 10 matings were recorded. All the tests were conducted in a room maintained at approximately 24°C under normal light conditions from 7:30 to 10:30 am. Three experiments were carried out, involving MY and y, PN and ca and TIR and ct. RESULTS Initially, we started our experiment to study rare type mating advantage for males. As a multiple-choice procedure was used, we were also able to examine mating advantage for rare females. As assortative mating may furnish a faulty rare type effect, these two components of mating success should be separated (Bryant et al., 1980; Kearns et al., 1990). Hence, we first analyzed the randomness of matings between the wild-type and mutant strains with pooled replicates. The c2 values from 2 x 2 contingency tests for assortative mating for all three pairs of strains were determined (Table 1). This approach reveals randomness in mating among females and males (Pot et al., 1980; Terzic et al., 1996). In these calculations it is presumed that matings are independent of the testing frequencies and genotypes. In the experiment involving MY and y flies, the 8:12 and 16:4 ratios showed significant preferential mating with the Ehrman method. In the latter case, wild-type flies showed preferential mating for their own type (P < 0.05). However, with the 8:12 ratio, though wild-type and mutant flies showed a tendency for homogamic matings, matings took place in all four combinations. Consequently, there was only one significant deviation (MY:y = 16:4), which is not enough to suggest preferential mating between these two strains. In the experiments with PN and ca and TIR and ct, significant deviations from random mating were seen in one case of 10 (P < 0.05, and P < 0.01, respectively); these can be ignored as isolated cases. However, one thing is common among these experiments (Table 1); three deviations were found when wild-type and mutant flies were placed in a 16:4 ratio. This is probably due to the fact that common flies have more access to the opposite sex of their own type at this ratio. On the whole, it can be concluded that there was no preferential mating encountered in any of the three pairs of strains. An isolation estimate (Table 1), a measurement of sexual isolation among two strains, was calculated by the formula of Merrell (1950):
If IE is 1, there is no sexual isolation between the strains. If IE is zero, then isolation is complete. There was no significant sexual isolation between the mutant and the wild-type strains (Table 1). The IE values with superscript ‘a’, with lower IE values (all were found at the 16:4 ratio) do not necessarily indicate existence of isolation between two strains, as no overall preferential mating between two strains was found by the 2 x 2 contingency analyses. This difference between homo- and heterogamic matings is due to the fact that at these lower ratios, common males mate much more with the common females due to their higher availability within the mating chamber. Also, it is clear that at these ratios, homogamic mating percentages for the common flies are higher than the homogamic mating percentages of the rare flies (percentages not shown) irrespective of their genotypes.
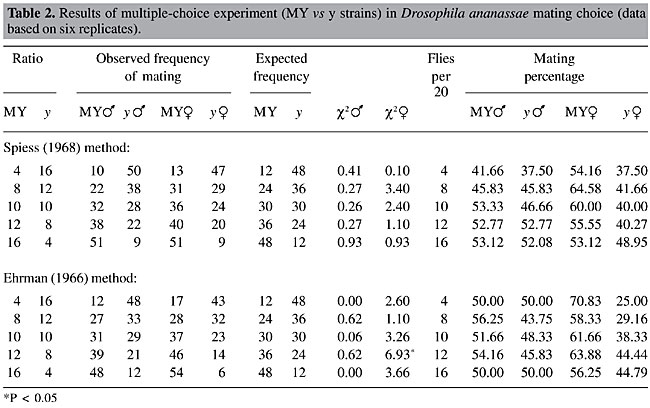
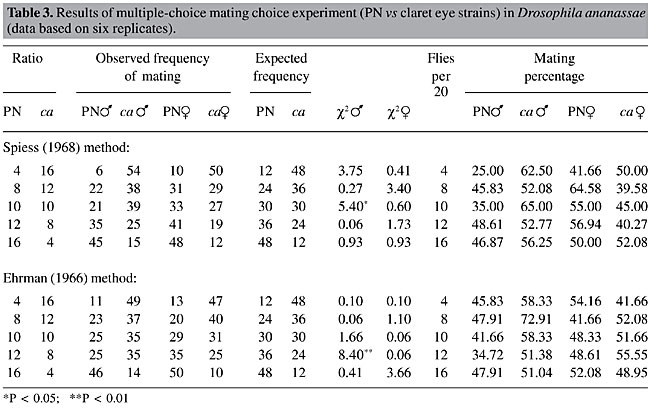
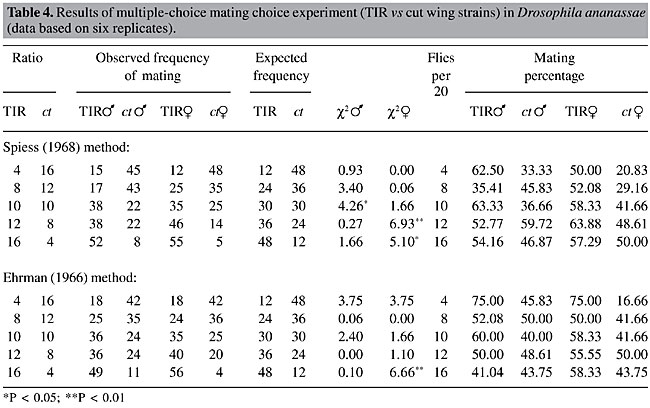
The results of direct observations following both methods were recorded for all three pairs of fly strains (Tables 2, 3 and 4). c2 values testing the mating success of two types of flies with respect to the input frequencies were calculated. The mating percentages of both the wild-type and mutant flies, as their ratios varied, were also calculated. The expected numbers of matings were calculated on the basis of the ratios between the two types of males or females introduced into the mating chamber. There were no significant differences between observed and expected number of matings of the two types of flies in the test with MY and y. There was a significant disadvantage for rare females (P < 0.01) at a single ratio (12 MY:8y), in the Ehrman method observations. Mating percentages also did not indicate any mating success for minority males or females. Rather, mating percentages were lower for mutant females (y), when tested with both methods, and they mated much less when they were rare in the Ehrman method. In the Spiess method observation with TIR and ct (Table 3), there was no significant rare type mating advantage for both males and females. Also, mating percentages did not show any particular trend. However, at the 10:10 ratio, the ca males were more successful than the wild-type males. In the test with TIR and ct, employing the Spiess method (Table 4), at the 10:10 ratio the wild-type males were more successful than the ct males. It is generally assumed that both types of males have equal mating ability if males are equally successful in mating when they are present at equal ratio (10:10) and can be treated as a ‘control’ for sexual activity, as we have found in with MY and y (Table 2), and with the Ehrman method for PN and ca (Table 3) and TIR and ct (Table 4). As we paired the strains for mating propensity, the deviations from the 10:10 ratios (Spiess method, Tables 3 and 4) were not due to differences in mating propensity, nor were they due to assortative mating or sexual isolation (Table 1). However, the Ehrman method did not reveal significant deviations from expected mating frequencies in the 10:10 presentation ratios (Tables 3 and 4). These deviations may be due to the different observation methods. In the females, random matings were found with the 10:10 ratios with both pairs of strains. In the experiment with PN and ca, employing the Ehrman method, ca males had a significant mating advantage (P < 0.01) when they were rare (PN: ca = 12:8). At other ratios, the advantage for rare type was not apparent, independent of the observation method. The rare female advantage was also not evident in the experiment with PN and ca. In the tests with TIR and ct, employing both observation methods, there was no rare type mating advantage for males or females. Rather, when ct females were rare, they were at a disadvantage in three cases of four (P < 0.05, P < 0.01). They had low mating percentages when they were rare (Table 4).
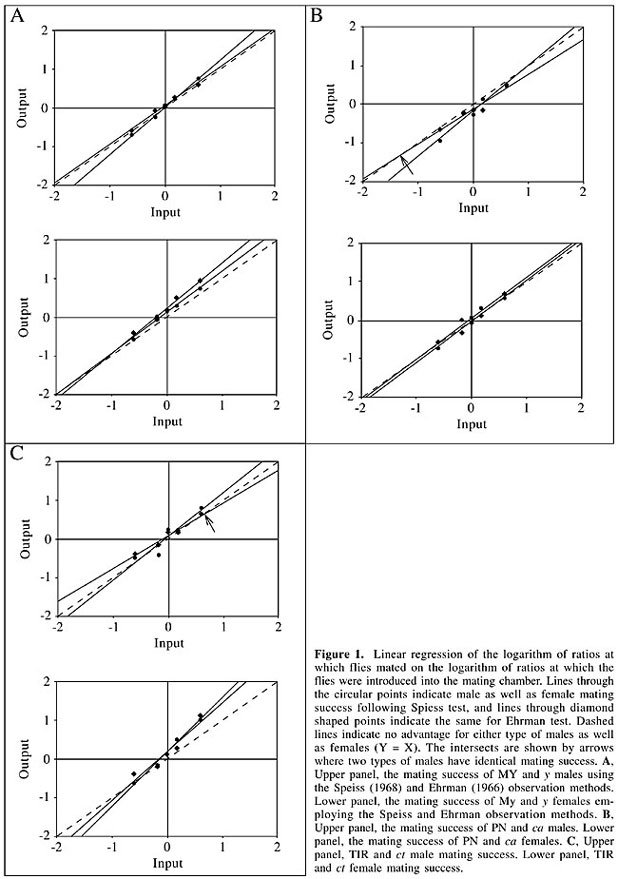
This approach to study frequency-dependent advantage, using c2 tests, has been discussed in the literature (Ayala, 1972; Adams and Duncan, 1979). The most serious drawback considered, was that by applying the c2 test, a test of differential fitness is made for each input frequency separately, while there is no test for a change in fitness over frequencies, which is the original reason for conducting the experiments. In view of this, Ayala (1972) and Ayala and Campbell (1974) proposed that it would be much more appropriate to study the overall fitness trend, by incorporating the outputs of all input frequencies in a single statistic. They suggested application of linear regression of logarithms of output on logarithms of input ratios to study rare type effects that may reveal rare type advantage, which might not be conspicuous in c2 tests. So, we analyzed our data by an ordinary regression equation,
DISCUSSION Ehrman et al. (1972) carried out frequency-dependent mating advantage tests using wild-type and y males of D. gaucha. Mutant males performed very poorly in comparison to the wild-type males, when they were common compared to the wild types or were equally abundant. Mutant flies performed better in competition with the wild type only when they were rare, in which case they were able to mate equally well as their wild-type counterparts. Sturtevant (1915) was the first to report that yellow males of D. melanogaster are usually unsuccessful in a competitive mating situation and that this is probably due to their reduced activity to stimulate the females. Bastock (1956) also found that yellow mutant males of D. melanogaster are less active than their wild-type counterparts. Later, in studies with D. melanogaster, Wilson et al. (1976) found that the yellow mutant has a reduction in body pigmentation associated with a decrement in locomotor activity and in male competitive mating activity. They indicated that the impaired locomotor activity of y males might not be the general cause of their lower mating speed and reduced competitive mating ability, as they found that the stimuli that y males provided to females were the same as those of wild-type males, both quantitatively and qualitatively. Also, Heisler (1984) remarked that low scores of y males in Wilson et al.’s (1976) work might be due to inbreeding. Earlier, Barker (1962) found by employing multiple-choice experiments that sexually matured wild type and y males mate almost equally with the y females. Threlkeld et al. (1974), who used female choice experiments, reported the development of female preference for y males in response to selection for enhanced acceptance of the y males, and concluded that it is misleading to regard y males as offering a low level of stimulus, without carefully defining the mating system. In our initial mating propensity test, we did not observe any reduced mating ability for males in our yellow strain of D. ananassae, rather it was quite high always, similar to the wild type. However, it was expected that y males would not show reduced competitive mating ability compared to the MY males as these lines were selected for approximately equal mating propensities. Neither of these two strains of D. ananassae had mating advantages when they were rare. Nor were the y males found to be less acceptable by the females. However, y females have been found to mate less than the wild-type females and they are at a disadvantage when they are rare. The reason that y females are less successful in comparison with wild-type females perhaps lie in the fact that the yellow locus has pleiotropic effects on the structure of the female genital apparatus, known from the work of Dobzhansky and Holz, 1943 (cited in Wilson et al., 1976). Later, Burnet and Connolly (1974) suggested possible involvement of yellow gene in the metabolic pathway of tyrosine to 3-4-dihydroxyphenylalanine, which is utilized in the biosynthetic pathways leading to the synthesis of sclerotonin and melanin; these substances are involved in the hardening and pigmentation of the cuticle. They suggested that mutation in the yellow gene causes a change in some mechanical properties of the integument, which may result in functional impairment of the genitalia. This may be the reason for our finding of mating disadvantage of mutant females, as males mate with y females less frequently, especially when they are rare, as more acceptable females are easily available (as males can also be choosy). Rare male experiments, using eye color mutants and wild-type strains, are not uncommon in the literature. Rare male advantage was first discovered in the white mutant (Petit, 1954). Thereafter, several research papers providing evidence for both advantages and disadvantages of rare eye color mutants were published (Knoppien, 1985a; Sondergaard, 1986; Spiess and Bowbal, 1987; Lichtenberger et al., 1988; Depiereux et al., 1990; Cakir and Kence, 1999). Both-sided rare male advantages were reported by Singh and Chatterjee (1989), who tested sepia (se) and cardinal (cd) eye color mutants against a VN-ST wild-type strain of D. ananassae. The ca eye color mutation that we used causes a reduced level of red and brown pigments. Many studies have evidenced the lower sexual fitness of eye color mutants, due to a positive correlation between pigment intensity and mating success, as vision plays a critical role in the courtship and mating of Drosophila (see Ochando, 1981), Just and Markow (1989) found almost equal mating success of vermilion mutant flies (sex-linked recessive mutation lacking brown pigment) with Canton-S wild-type flies at equal ratios (1:1). In our experiment, ca males of D. ananassae did not show lower mating success, even when in competition with the wild-type flies. Instead, one-sided rare male mating advantage was found for rare ca males. Since randomization was carried out to remove the differences in the residual genetic background outside the ca locus in both strains, it is likely that the advantage of ca males is a function of the mutant phenotype. Though rare ca females did not have a mating advantage, they were not at a disadvantage in mating in competition with wild-type females. Ochando (1981), based on quantitative analysis of eye pigments in mutants of Drosophila, reported that eye pigmentation is not necessarily related to visual ability. He indicated that the visual process is extremely complex and visual acuity is not necessarily directly related to greater pigment content (ommochromes and pteridines). Consequently, it can be concluded that the ca eye color mutation does not weaken the mutant males due to lower visual ability, nor does it prevent the females from accepting visual stimuli from the males. We previously found evidence for both-sided rare male mating advantage using wild-type and ct wing strains of D. bipectinata (Singh and Sisodia, 1997) in a female-choice test. In the present experiment, using wild-type and cut wing flies of D. ananassae, a one-sided rare male mating advantage was found for TIR males, though rare ct males were not privileged; this was not found in the individual ratios, employing the c2 test, but was revealed when mating success was compared for changing ratios in a single statistic (Table 5). TIR and ct flies had approximately equal mating propensities, and no impairment in the mating ability of mutant males was observed. Earlier, Singh and Sisodia (1996) compared the mating ability of wild-type and ct wing flies of D. bipectinata and found that the wild- and mutant type strains were equally successful in mating, which is in agreement with what we found here with strains of D. ananassae. The ability to distinguish a rare from a common type implies discriminatory capacities (Ehrman, 1990). At the same time, another established fact is that the mate recognition system is polygenic in Drosophila. So, it is difficult to say exactly what caused this one-sided minority advantage for TIR males. Still, it can be said, since randomization was done in the genetic background outside the cut locus, that this advantage is related to this locus. Minority advantage for TIR females was absent and mutant females had disadvantages when they were rare. In Drosophila, cut wing is a complex locus and is known for its pleiotropic effects. It was found in D. melanogaster that much of this locus is devoted to tissue- and stage-specific activation of the structural element (Johnson and Judd, 1979). This may somehow makes ct females less receptive to both the males and thus disadvantaged when they are in a minority. Anderson and McGuire (1978) concluded that “Mating success is probably important as a component of fitness in both sexes, but in most experiments it can be determined accurately only for males”. Rare female mating advantage has not been discussed in the literature as exhaustively as it has been for males, though a few references are available regarding this aspect (Knoppien, 1985a; Cereghetti et al., 1987; Lichtenberger et al., 1988; Depiereux et al., 1990; Dernoncourt-Sterpin et al., 1991; Singh and Sisodia, 2000). In our experiment, a rare female mating disadvantage was found in the case of y and ct females, both sex-linked recessive mutations, but not in the ca females (autosomal). In Drosophila males, X-linked genes are hyperactivated, by which means total X-linked gene activity in male and female is approximately equalized. However, this is not always true, e.g., we observed failure of wing expansion more frequently in females than in males in the ct strain. So, it is quite possible that degree of expression of pleiotropic effects of y and ct loci differs in males and females, which might have led to minority mating disadvantages in females but not in males in the sex-linked mutants. In our experiment, two observational methods were employed, the Spiess method and the Ehrman method (see Material and Methods). The Spiess method, in which mating pairs are aspirated out, furnishes a minimal value for rare male mating advantage, as there is no chance of remating in males (females generally do not remate as quickly). But it is clear that as copulating pairs are aspirated out, changes in the initial ‘relative’ sex ratio (ratio of X male to Y females or Y males to X females) occur. However, Dernoncourt-Sterpin et al. (1991) showed that the relative sex ratio factor plays only a very minor role in determining the rare type advantage found in the genotype ratio experiments. However, one male can take advantage of the courtship stimulation of another male type when both forms are courting the same females (Chatterjee and Singh, 1989). In these strains of D. ananassae, one or more males could often be observed surrounding a copulating pair. So, removing the copulating pair may disturb the second male’s sexual activity. This may affect the outcome of a rare male experiment. On the contrary, in the Ehrman method, as copulating flies are not removed, rare males may gain much of their advantage from the possibility of mating more than once (Knoppien, 1985a). However, this should not be considered as a source of erroneous results showing strong rare male mating advantage, as repeated matings reinforce sexual selection, favoring males that mate repeatedly (Singh and Singh, 2001). In our experiment, rare male advantages as well as rare female disadvantages were more prominent with the Ehrman method than with the Spiess method. So the Ehrman method appears to be a more effective measure than the Spiess method. However, based on the ANOVA analysis, differences between the two methods did not significantly affect the outcome of the rare male experiments. In summary, we found a one-sided rare male mating advantages for an eye color mutant (ca) and for a wild-type strain of D. ananassae in two different experiments, giving further evidence in support of minority-mating advantage and indicating influence of a mutant locus on this phenomenon. However, this could not be detected in another experiment employing wild-type flies and yellow flies. This, and one-sided male mating success in two other experiments indicate that this might not be a universal phenomenon in Drosophila and perhaps it is not the only mechanism maintaining genetic polymorphisms in Drosophila. Moreover, we found minority mating disadvantages for rare females in y and ct strains, though they performed well when they were common or in equal proportion with the wild-type females in the mating chamber. To our knowledge, this is the first report for rare female mating disadvantage in Drosophila, though a disadvantage for rare males was reported earlier by Peterson and Merrell (1983). We found all these non-random matings in the absence of assortative mating or sexual isolation or a difference in mating propensities between two experimental strains, which, in agreement with Bryant et al. (1980), we think should be ensured to demonstrate a real minority effect. Moreover, our results indicate that application of different observational methods do not significantly alter the outcome of frequency-dependent experiments. Finally, the mechanism of rare type advantage is not clearly understood. On the presumption that female choice is the key factor for producing a rare male advantage, different models regarding minority-mating advantage have been postulated, e.g., the sampling and habituation hypothesis (Ehrman and Spiess, 1969), the avoidance hypothesis (Spiess and Kruckeberg, 1980), constant female preference for one male type (O’Donald, 1977), and female discrimination capacity among different male phenotypes (Spiess and Bowbal, 1987). However, a female’s ability to discriminate rare males is not fully understood, as Ayala and Campbell (1974) said that “When the whole genome is considered, every individual Drosophila has a unique genotype and thus is a rare type. Moreover, it is not likely that every single gene difference could be “recognized” by the flies. Yet either the females or the males, or both, must recognize a difference for the females to identify the rare males and prefer them as mates, or for the rare-type males to become sexually more active.” Another major factor in mate recognition has been highlighted by Cobb and Ferveur (1996), “One of the major problems that bedevil behavior genetic studies is pleiotropy, or the production of multiple phenotypic effects from one genotypic effect. ........for studies that seek to pin down single, unitary effects, however, pleiotropy can lead to erroneously simple interpretations.” A more recent concern of male choice for their females (Noor, 1996; Blows and Allan, 1998; Van Gossum, 2000; Bonduriansky, 2001) is expected to be included in minority-mating advantage experiments in the future. ACKNOWLEDGMENTS Financial assistance in the form of JRF of CAS, Department of Zoology, BHU to A. Som is gratefully acknowledged. The authors thank Prof. M. Matsuda, Kyorin University, Tokyo, Japan, for providing the mutant stocks. REFERENCES Adams, W.T. and Duncan, G.T. (1979). A maximum likelihood statistical method for analyzing frequency-dependent fitness experiments. Behav. Genet. 9: 7-21. Anderson, W.W. (1969). Polymorphism resulting from the mating advantage of rare male genotypes. Proc. Natl. Acad. Sci. USA 64: 190-197. Anderson, W.W. and McGuire, P.R. (1978). Mating pattern and mating success of Drosophila pseudoobscura karyotypes in large experimental populations. Evolution 32: 416-423. Ayala, F.J. (1972). Frequency-dependent mating advantage in Drosophila. Behav. Genet. 2: 85-91. Ayala, F.J. and Campbell, C.A. (1974). Frequency-dependent selection. Annu. Rev. Ecol. Syst. 5: 115-138. Ball, S.J., Adams, M., Possingham, H.P. and Keller, M.A. (2000). The genetic contribution of single male immigrants to small, inbred populations: a laboratory study using Drosophila melanogaster. Heredity 84: 677-684. Barker, J.S.F. (1962). Studies of selective mating using the yellow mutant of Drosophila melanogaster. Genetics 47: 623-640. Bastock, M. (1956). A gene mutation which changes a behaviour pattern. Evolution 10: 421-439. Blows, M.W. and Allan, R.A. (1998). Levels of mate recognition within and between two Drosophila species and their hybrids. Am. Nat. 6: 826-837. Bonduriansky, R. (2001). The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol. Rev. 76: 305-339. Bryant, E.H., Kence, A. and Kimball, K.T. (1980). A rare-male advantage in the housefly induced by wing clipping and some general considerations for Drosophila. Genetics 96: 975-993. Burnet, B. and Connolly, K. (1974). Activity and sexual behaviour in Drosophila melanogaster. In: The Genetics of Behaviour (van Abeelen, J.H.F., ed.). Amsterdam, North-Holland. Cakir, S. and Kence, A. (1999). Lack of minority advantage in Drosophila melanogaster mutants. Turk. J. Biol. 23: 433-443. Casares, P., Carracedo, M.C., Del Rio, B., Pineiro, R., Garcia-Florez, L. and Barros, A.R. (1998). Disentangaling the effects of mating propensity and mating choice in Drosophila. Evolution 52: 126-133. Cereghetti, M., Jacquemin, F., Hols, P., Lichtenberger, M. and Elens, A. (1987). Measurement of frequency-dependent sexual activity in Drosophila melanogaster. Genetica 75: 167-171. Chatterjee, S. and Singh, B.N. (1989). Rare male mating advantage. Cell Biol. Newsletter 11: 29-34. Cobb, M. and Ferveur, J.F. (1996). Evolution and genetic control of mate recognition and stimulation in Drosophila. Behav. Proc. 35: 35-54. Dal Molin, C. (1979). An external scent as the basis for a rare-male mating advantage in Drosophila melanogaster. Am. Nat. 113: 951-954. Depiereux, E., Dernoncourt-Sterpin, C., Lechein, J., Feytmans, E. and Elens, A. (1990). Direct observation of sexual competition in Drosophila melanogaster: The mutant white in competition with other genotypes. Behav. Genet. 20: 511-533. Dernoncourt-Sterpin, C., Lechien, J. and Elens, A. (1991). Sex ratio, relative frequency, and mating success in two genotypes of Drosophila melanogaster. Behav. Genet. 21: 471-485. Dobzhansky, Th. and Holz, A.M. (1943). A re-examination of the problem of manifold effects of genes in Drosophila melanogaster. Genetics 28: 295-303. Ehrman, L. (1966). Mating success and genotype frequency in Drosophila. Anim. Behav. 14: 332-339. Ehrman, L. (1990). Developmental isolation and subsequent adult behaviour of Drosophila pseudoobscura. Behav. Genet. 20: 609-615. Ehrman, L. and Kim, Y.-K. (1995). Influence of developmental isolation on Drosophila paulistorum rare male mating advantages. Dros. Inf. Serv. 76: 125-126. Ehrman, L. and Spiess, E.B. (1969). Rare-type mating advantage in Drosophila. Am. Nat. 103: 675-680. Ehrman, L., Koref-Santibanez, S. and Falk, C.T. (1972). Frequency dependent mating in two species of the mesophragmatica species groups of Drosophila. Dros. Inf. Serv. 48: 36-37. Elens, A.A. and Wattiaux, J.M. (1964). Direct observation of sexual isolation. Dros. Inf. Serv. 39: 118-119. Grant, B., Burton, S., Contoreggi, C. and Rothstein, M. (1980). Outbreeding via frequency-dependent mate selection in the parasitoid wasp, Nasonia (= Mormoniella) vitripennis Walker. Evolution 34: 983-992. Heisler, I.L. (1984). Inheritance of female mating propensities for yellow locus genotypes in Drosophila melanogaster. Genet. Res. 44: 133-149. Johnson, T.K. and Judd, B.H. (1979). Analysis of the cut locus of Drosophila melanogaster. Genetics 92: 485-502. Just, J. and Markow, T. (1989). Success of mutant Drosophila at different sex ratios. Hereditas 110: 51-53. Kearns, P.W.E., Tomlinson, I.P.M., O’Donald, P. and Veltman, C.J. (1990). Non-random mating in the two-spot lady bird (Adalia bipunctata): I. A reassessment of the evidence. Heredity 65: 229-240. Knoppien, P. (1985a). Rare male mating advantage: a review. Biol. Rev. 60: 81-117. Knoppien, P. (1985b). The numbers of males stored per vial, a possible source of bias in rare male experiments. Dros. Inf. Serv. 61: 101. Knoppien, P. (1987). Rare-male mating advantage: An artifact caused by differential storage conditions? Behav. Genet. 17: 409-425. Lewontin, R.C. (1974). The Genetic Basis of Evolutionary Change. Columbia University Press, New York. Lichtenberger, M., Lechien, J. and Elens, A. (1988). The frequency dependence of mating success in Drosophila melanogaster. Genetica 77: 25-52. Markow, T.A. (1980). Rare-male advantages among Drosophila of the same laboratory strain. Behav. Genet. 10: 553-555. Merrell, D.J. (1950). Measurement of sexual isolation and selective mating. Evolution 4: 326-331. Noor, M.A.F. (1996). Absence of species discrimination in Drosophila pseudoobscura and Drosophila persimilis males. Anim. Behav. 52: 1205-1210. Ochando, M.D. (1981). Mating behaviour and analysis of eye pigmentation of several mutants of Drosophila melanogaster. Genetica 55: 117-121. O’Donald, P. (1977). Mating advantage of rare males in models of sexual selection. Nature 267: 151-154. Partridge, L. (1988). The rare-male effect: what is its evolutionary significance? Philos. Trans. R. Soc. Lond. B 319: 525-539. Peterson, J.R. and Merrell, D.J. (1983). Rare male mating disadvantage in Drosophila melanogaster. Evolution 37: 1306-1316. Petit, C. (1951). Le role de lisolment sexuel dans levolution des populations de Drosophila melanogaster. Bull. Biol. Fr. Belg. 85: 392-418. Petit, C. (1954). L’isolement sexuel chez Drosophila melanogaster. Etude du mutant white et de son alle’lomorphe sauvage. Bull. Biol. 88: 435-443. Pot, W., Van Delden, W. and Kruijt, J.P. (1980). Genotypic differences in mating success and the maintenance of the alcohol dehydrogenase polymorphism in Drosophila melanogaster: No evidence for over dominance or rare genotype mating advantage. Behav. Genet. 10: 43-58. Sequeira, W., Nelson, C.R. and Szanter, P. (1989). Genetic analysis of the claret locus of Drosophila melanogaster. Genetics 123: 511-524. Singh, B.N. (1996). Population and behaviour genetics of Drosophila ananassae. Genetica 97: 321-329. Singh, B.N. (2000). Drosophila ananassae: a species characterized by several unusual genetic features. Curr. Sci. 78: 391-398. Singh, B.N. and Chatterjee, S. (1989). Rare-male mating advantage in Drosophila ananassae. Genet. Sel. Evol. 21: 447-455. Singh, B.N. and Singh, S.R. (2001). Female remating in Drosophila ananassae: Evidence for sperm displacement and greater productivity after remating. Zool. Sci. 18: 181-185. Singh, B.N. and Sisodia, S. (1996). No effect of cut wing mutation on mating propensity in Drosophila bipectinata. Biol. Zentralbl. 115: 46-50. Singh, B.N. and Sisodia, S. (1997). Evidence for rare-male mating advantage in Drosophila bipectinata. Genetika 29: 41-48. Singh, B.N. and Sisodia, S. (2000). Frequency-dependent selection: minority male mating advantage in Drosophila. Curr. Sci. 78: 141-150. Singh, B.N. and Som, A. (2001). Evidence for rare male mating advantage and sexual isolation in two karyotypically different strains of Drosophila ananassae. Curr. Sci. 81: 1473-1477. Som, A. and Singh, B.N. (2002). No evidence for minority male mating advantage in wild-type strains of Drosophila ananassae tested in multiple-choice experiments. Genet. Mol. Res. 1: 317-326. Som, A. and Singh, B.N. (2004). Rare male mating advantage for inversion karyotype of Drosophila ananassae. Behav. Genet. 34: 335-342. Sondergaard, L. (1986). Mating competition in artificial populations of Drosophila melanogaster polymorphic for ebony II. A test for minority male mating advantage. Genet. Res. 47: 205-208. Spiess, E.B. (1968). Low frequency advantage in mating of Drosophila pseudoobscura karyotypes. Am. Nat. 102: 363-379. Spiess, E.B. and Bowbal, D.A. (1987). Minority mating advantage of certain eye color mutants of Drosophila melanogaster. IV. Female discrimination among three genotypes. Behav. Genet. 17: 291-306. Spiess, E.B. and Kruckeberg, J.F. (1980). Minority advantage of certain eye color mutants of Drosophila melanogaster. II. A behavioral basis. Am. Nat. 115: 307-327. Spieth, H.T. (1966). Mating behaviour of D. ananassae and ananassae-like flies from the Pacific. Univ. Tex. Publ. 6615: 133-145. Sturtevant, A.H. (1915). Experiments on sex recognition and the problem of sexual selection in Drosophila. J. Anim. Behav. 5: 351-366. Terzic, T., Andjelkovic, M., Marinkovic, D. and Stamenkovic-Radak, M. (1996). Frequency dependent selection: I. Rare male phenomenon in Drosophila subobscura dependent on the proportion of Amy genotypes and substrate composition. J. Evol. Biol. 9: 337-355. Threlkeld, S.F.H., Procwat, R.A., Abbot, K.S. and Yeung, A.D. (1974). Genetically based behaviour patterns in Drosophila melanogaster. Nature 247: 232-233. Tobari, Y.N. (Ed.) (1993). Drosophila ananassae. Genetical and Biological Aspects. Japan Scientific Societies Press, Tokyo, Japan. Van Gossum, H., Stoks, R. and De Bruyn, L. (2000). Reversible frequency-dependent switches in male mate choice. Proc. R. Soc. Lond. B 268: 83-85. Wilson, R., Burnet, B., Easrtwood, L. and Connolly, K. (1976). Behavioural pleiotropy of the yellow gene in Drosophila melanogaster. Genet. Res. 28: 75-88. |
|