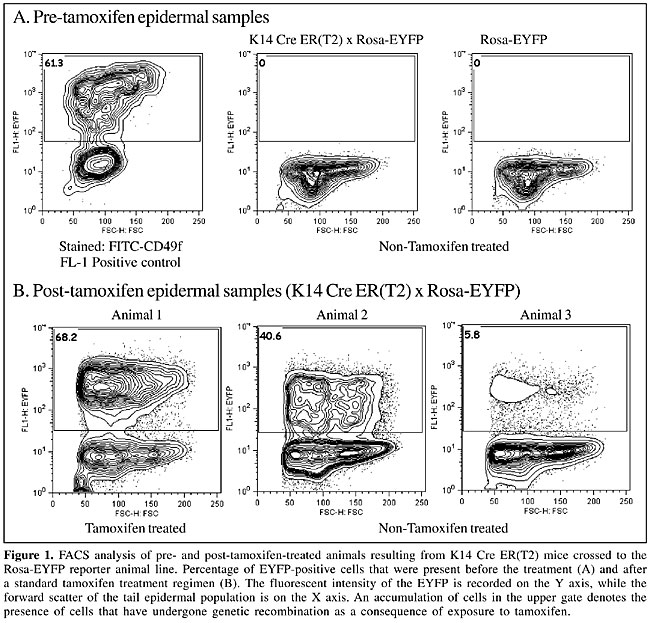
ABSTRACT. Inducible transgenic mouse models that impose a constraint on both temporal and spatial expression of a given transgene are invaluable. These animals facilitate experiments that can address the role of a specific cell or group of cells within an animal or in a particular window of time. A common approach to achieve inducibility involves the site-specific recombinase ‘Cre’, which is linked to a modified version of one of various steroid hormone-binding domains. Thus, the expression of Cre is regulated such that a functional nuclear transgene product can only be generated with the addition of an exogenous ligand. However, critical requirements of this system are that the nuclear localization of the transgene product be tightly regulated, that the dosage of the inducing agent remains consistent among experimental animals and that the transgene cassette cannot express in the absence of the inducing agent. We used the Cre ER(T2) cassette, which is regulated by the addition of the estrogen antagonist tamoxifen to determine whether cross-contamination of tamoxifen between animals housed together can be a significant source of spurious results. We found that cross-contamination of exogenous tamoxifen does occur. It occurred in all animals tested. We suggest that the mechanism of contamination is through exposure to tamoxifen in the general environment and/or to coprophagous behavior. These results have important implications for the interpretation and design of experiments that use ‘inducible’ transgenic animals. Key words: Tamoxifen, Estrogen receptor, Inducible transgenic, Cre, Hormone-binding domain, ER(T2) INTRODUCTION Numerous transgenic mouse models have suffered from the inability to temporally control the expression of the transgene of interest. This has led to wide spread use of the P1 phage-derived Cre-recombinase system (Sternberg, 1981; Sternberg and Hamilton, 1981; Sternberg et al., 1981). This integrase is capable of mediating an excision recombination event between two flanking LoxP DNA sequences, resulting in gene deletion or transgene activation. More recently, additional control has been provided by cytoplasmic versus nuclear localization of Cre recombinase. The first of these approaches was implemented by Gossen and colleagues (Gossen and Bujard, 1992), who have pioneered the tetracycline trans-activator (tTA) cassette, which is still used frequently in both in vitro and in vivo experiments. An alternative strategy for temporal control of Cre involves linking the coding sequence of the Cre gene to the hormone-binding domain of one of several steroid hormone receptors. These receptors are generally engineered such that exogenous administration of the appropriate ligand results in the transgene product relocating into the nucleus. Various steroid hormone receptors have been targeted by this strategy, including the glucocorticoid receptor (Brocard et al., 1998a), the progesterone receptor (Kellendonk et al., 1996) and the estrogen receptor of both humans (ER(T)) (Feil et al., 1996, 1997) and mice (ER(TM)) (Danielian et al., 1998). In the estrogen receptor models, an additional layer of regulation is provided, as nuclear translocation occurs with the synthetic estrogen antagonist tamoxifen, but not with the endogenous estrogen receptor ligands. This model has been employed in several transgenic lines and has the advantage of being tightly regulated and very efficient in the target tissue. Drawbacks include the relatively slow response in gene expression following the cessation of therapy (Brocard et al., 1998b) and the complexities associated with delivering an oil-soluble ligand. Subsequent to the generation of the modified hormone-binding domain of the human estrogen receptor (ER(T)) (Feil et al., 1996), Indra and colleagues produced a second generation mutant ER(T2) (Indra et al., 1999), which gives a 4-fold increase in the efficiency of recombination induced by 4-hydroxy-tamoxifen in cultured cells. When placed under the CMV promoter, this Cre ER(T2) transgene produces a high level of recombination, in numerous tissues, with the highest level (40%) being observed in the skin (Metzger and Chambon, 2001). The potency of these inducible transgenic animal lines, when coupled to robust reporter lines, has provided tools to address complex questions about cell origin and migration. However, an essential requirement of these animal lines is that they do not demonstrate aberrant Cre expression in the absence of the exogenous ligand. This would substantially confound the interpretation of experimental results. We designed experiments to determine if ‘cross-contamination’ of untreated littermates that were housed with tamoxifen-treated animals were a source of spurious results in the untreated animals. The spurious activation of a reporter gene in control animals is a potential mechanism by which confusing experimental data can be generated. MATERIAL AND METHODS Tail biopsy Tail biopsies were taken and single cell suspensions of the tail epidermis prepared. For the ‘Pre-tamoxifen’ biopsies, 5 mm of the tail tip was removed. The tail samples were washed in cold sterile PBS, the skin was isolated and all tail pieces were then digested in 5 ml of neutral dispase II (8 mg/ml, Roche), overnight at 4°C, to dissolve the dermal-epidermal junction attachments. After overnight digestion, the tail biopsies were rinsed in cold PBS and the epidermis separated from the dermis. The peeled epidermis was washed in fresh PBS and was cut into 3-4 small pieces. The epidermis was drained and digested using 1X trypsin-EDTA (Thermo Trace Ltd.) at 37°C at 500 rpm for exactly 4 min. After digestion, the vessel was immediately placed in ice and the reaction quenched with an equal volume of cold soybean trypsin inhibitor (Sigma, USA). The epidermal slurry was then filtered through a 70-µm, and then a 40-µm cell strainer. The cell pellet was then centrifuged at 400 g for 5 min at 4°C, after which the cells were resuspended, counted and subsequently prepared for FACS analysis. All samples gave a viability of no less than 70%, as assessed by trypan blue exclusion. Tamoxifen administration Animals treated with tamoxifen received a 5-day course of tamoxifen (Sigma, USA). The tamoxifen was delivered intraperitoneally at a standard dosage of 1 mg/day (Indra et al., 1999). This dose was prepared as previously described (Indra et al., 1999), with the exception that only corn oil was used for resuspension of the tamoxifen (Hayashi and McMahon, 2002). Flow cytometry analysis Epidermal cells were analyzed by flow cytometry using a FACS caliber (Becton Dickinson) for analysis. Dead cells were eliminated from the analysis by propidium iodide exclusion. Due to the small size of the “pre-tamoxifen” tissue biopsy, only 5,000 cells were analyzed per sample, while in post-tamoxifen samples, 30,000 cells per tail were analyzed. As detection of enhanced yellow fluorescent protein (EYFP) was used as a measure of the efficiency of Cre deletion, mediated by tamoxifen administration, a stained positive control was used to set up the flow cytometer. A FITC-labeled antibody directed against the a6 integrin (CD49f, Becton Dickinson) was used for this purpose. RESULTS Analysis of recombination in the absence of tamoxifen treatment To assess the potential for cross-contamination between littermates housed together, genetic crosses were performed between hemizygous K14 Cre ER(T2) (Indra et al., 2000) and homozygous Rosa-EYFP (Srinivas et al., 2001) reporter mice. One litter of 4-week-old K14 Cre ER(T2) x Rosa-EYFP mice was double tagged by ear punch and toe tagging to prevent misidentification. The genotyping of this litter (n =11) revealed four double-positive transgenic animals. The epidermal cells were analyzed for endogenous expression of EYFP by flow cytometry. Expression of EYFP reflected the number of cells within the preparation that had been exposed to tamoxifen and had therefore recombined their DNA to remove the “stop” cassette, which otherwise prevented expression of the EYFP. The positive control gave significant staining in the FL-1 channel (Figure 1A, plot 1). In contrast, there was no detection of EYFP in all of the control animals (Figure 1A, plots 2 and 3), confirming that there was no background recombination in the absence of tamoxifen treatment.
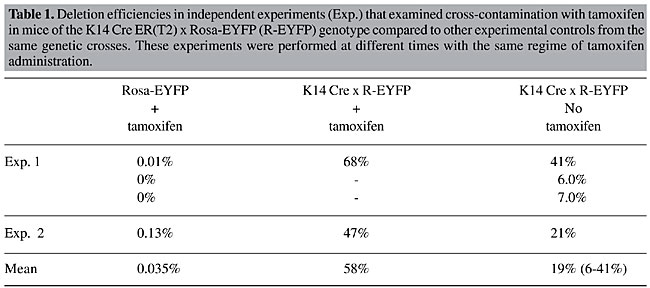
Standard tamoxifen administration results in varied amounts of in vivo tissue deletion In the litter analyzed ‘pre-tamoxifen’, half of the animals were subsequently treated with a 5-day course of tamoxifen. The remaining animals were not treated but were housed with their tamoxifen-treated littermates. On day 7 the experiment was terminated and a second tail epidermal sample was taken. These samples were treated as above, and 30,000 cells were analyzed by flow cytometry (Figure 1B). The K14 Cre ER(T2) x Rosa-EYFP transgenic mice, when treated with tamoxifen, generated efficient recombination in the epidermal fraction of the skin biopsy, as measured by EYFP expression. About two thirds (68.2%) of the epidermal cells expressed EYFP (Figure 1B, plot 1). In contrast, “control” animals within the group of untreated double-transgenic animals demonstrated varying but substantial levels of DNA deletion, with consequent EYFP expression. This was interpreted as cross-contamination of tamoxifen between littermates. There was a range of DNA deletion and EYFP expression, which varied from 6-40% of total epidermis (Figure 1B, plots 2 and 3). This variation is probably reflective of the extent to which these animals were exposed to the tamoxifen present in their environment. To eliminate the possibility of potential error triggered by poor animal labeling or other technical errors, a repeat analysis was performed on a second litter where there was only one, double-transgenic animal present in each of the treated and untreated groups (Table 1). A similar level of cross-contamination was observed. A deletion efficiency of 47% was observed in the tamoxifen-treated double-transgenic animal, whereas in the untreated animal inadvertent cross-contamination led to the deletion of 21% in the cells in the epidermal fraction.
DISCUSSION Combined, these results suggest that animals can cross-contaminate each other following exposure to intraperitoneally administered tamoxifen. However, there was considerable variation in the amount of contamination that occurred. On average, the inadvertent cross-contamination occurred with a range of efficiency of 9-60% of the intentionally treated animals. Despite this variation, there was some level of deletion mediated by cross-contamination by tamoxifen in all double-transgenic animals. The mechanism of cross-contamination was not elucidated. However, tamoxifen is an oil-soluble compound that is readily absorbed through the dermis (Vasioukhin et al., 1999). It is likely that the injected tamoxifen leaked from the injection site in treated animals and/or was excreted either renally or gastrically in treated animals, and this served as a source for cross-contamination. In addition, coprophagous behavior has been documented in mice from as young as 14 days, and it continues throughout adulthood (Ebino et al., 1987). Inadvertent cross-contamination of tamoxifen-treated animals occurred when animals were housed together. These findings are of particular significance in experiments where neonatal animals (preweaning) are required for analysis. Similarly, when a new transgenic line is being assessed, the efficiency of deletion at all ages of development is a normal line of enquiry. These experiments can easily be confounded by this phenomenon of cross-contamination. Another common practice is to determine the efficacious dose of tamoxifen by administering increasing concentrations of this ligand to informative, double-transgenic animals. This is often done with animals from the same litter. Cross-contamination may also have implications for this type of analysis. Furthermore, the results have implications for other methods of tamoxifen dosing in these inducible transgenic models. For example, several groups have utilized oral pellets in the delivery of tamoxifen. These pellets are a convenient way to deliver a long-term dose of tamoxifen. However, consumption of the pellets can produce minute particles that can become airborne and potentially could lead to cross-contamination of animals, even when they are housed in separate cages. The inference from the data presented here supports the use of micro-isolator chambers to house inducible transgenic animals being treated with oral tamoxifen pellets to reduce the risk of cross-contamination between animals. Ligand-dependent site-specific recombinases provide a powerful tool to engineer the mouse genome in specific somatic cell types and selected times in development. However, the possible effect of the confounding phenomena described here should influence experimental design. ACKNOWLEDGMENTS Research supported in part by grants from the National Health and Medical Research Council, Canberra, Australia and the National Institute for Health, USA. We thank Dr. D. Izon and Mr. R. Redvers for technical assistance. We also thank Professor Pierre Chambon for kindly supplying the K14-Cre mice and Dr. S. Sirnivas for supplying the Rosa EYFP reporter mice. REFERENCES Brocard, J., Feil, R., Chambon, P. and Metzger, D. (1998a). A chimeric Cre recombinase inducible by synthetic, but not by natural ligands of the glucocorticoid receptor. Nucleic Acids Res. 26: 4086-4090. Brocard, J., Warot, X., Wendling, O., Messaddeq, N., Vonesch, J.L., Chambon, P. and Metzger, D. (1998b). Spatio-temporally controlled site-specific somatic mutagenesis in the mouse. Proc. Natl. Acad. Sci. USA 94: 14559-14563. Danielian, P.S., Muccino, D., Rowitch, D.H., Michael, S.K. and McMahon, A.P. (1998). Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 8: 1323-1326. Ebino, K.Y., Suwa, T., Kuwabara, Y., Saito, T.R. and Takahashi, K.W. (1987). Lifelong coprophagy in male mice. Jikken Dobutsu 36: 273-276. Feil, R., Brocard, J., Mascrez, B., LeMeur, M., Metzger, D. and Chambon, P. (1996). Ligand-activated site-specific recombination in mice. Proc. Natl. Acad. Sci. USA 93: 10887-10890. Feil, R., Wagner, J., Metzger, D. and Chambon, P. (1997). Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem. Biophys. Res. Commun. 237: 752-757. Gossen, M. and Bujard, H. (1992). Tight control of gene expression in mammalian cells. Proc. Natl. Acad. Sci. USA 89: 5547-5551. Hayashi, S. and McMahon, A.P. (2002). Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 244: 305-318. Indra, A.K., Warot, X., Brocard, J., Bornert, J.M., Xiao, J.H., Chambon, P. and Metzger, D. (1999). Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 27: 4324-4327. Indra, A.K., Li, M., Brocard, J., Warot, X., Bornert, J.M., Gerard, C., Messaddeq, N., Chambon, P. and Metzger, D. (2000). Targeted somatic mutagenesis in mouse epidermis. Horm. Res. 54: 296-300. Kellendonk, C., Tronche, F., Monaghan, A.P., Angrand, P.O., Stewart, F. and Schutz, G. (1996). Regulation of Cre recombinase activity by the synthetic steroid RU 486. Nucleic Acids Res. 24: 1404-1411. Metzger, D. and Chambon, P. (2001). Site- and time-specific gene targeting in the mouse. Methods 24: 71-80. Srinivas, S., Watanabe, T., Lin, C.S., William, C.M., Tanabe, Y., Jessell, T.M. and Costantini, F. (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1: 4. Sternberg, N. (1981). Bacteriophage P1 site-specific recombination. III. Strand exchange during recombination at lox sites. J. Mol. Biol. 150: 603-608. Sternberg, N. and Hamilton, D. (1981). Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J. Mol. Biol. 150: 467-486. Sternberg, N., Hamilton, D. and Hoess, R. (1981). Bacteriophage P1 site-specific recombination. II. Recombination between loxP and the bacterial chromosome. J. Mol. Biol. 150: 487-507. Vasioukhin, V., Degenstein, L., Wise, B. and Fuchs, E. (1999). The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc. Natl. Acad. Sci. USA 96: 8551-8556. |
|