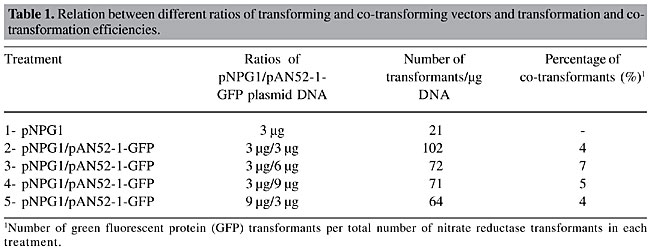
ABSTRACT. Penicillium griseoroseum, a deuteromycete fungus producer of pectinolytic enzymes, was transformed with a gene encoding for green fluorescent protein (GFP). The selection of transformants was based on the homologous nitrate reductase gene (niaD). Protoplasts of a P. griseoroseum Nia mutant (PG63) were co-transformed with the plasmids pNPG1 and pAN52-1-GFP. The plasmid pNPG-1 carries the homologous niaD gene and pAN52-1-GFP carries the SGFP-TYG version of GFP. The highest transformation efficiency (102 transformants/µg of pNPG1) resulted from the utilization of equimolar amounts of transforming and co-transforming vectors. Analysis of pAN52-1-GFP insertions into the genomic DNA of the transformants revealed single and multiple copy integrations. The transformants possessing a single copy of the gfp gene showed a low level of fluorescence, whereas multicopy transformants displayed strong fluorescence under visualization with fluorescent light. The transformants showing high expression of the gfp gene had the normal mycelia pigmentation altered, displaying a bright green-yellowish color, visible with the naked eye on the plates, without the aid of any kind of fluorescent light or special filter set. Key words: Penicillium griseoroseum, Transformation, Protoplasts, GFP, Pectinases, Mutant INTRODUCTION Fungi are important organisms for the industrial production of a broad range of extracellular enzymes. Pectinolytic enzymes, such as polygalacturonase and pectin lyase, play a key role in biotechnological processes of the textile and food industries. In the textile industries, they are used for degumming fibers, a process with a lower environmental impact than the chemical process (for a review, see Kashyap et al., 2001). The food industries utilize the pectinolytic enzymes in biotechnological processing of beverages, such as wine and fruit juices (Kawano et al., 1999). Fungi of the genus Penicillium are among the microorganisms that produce this enzymatic complex. Screening performed to assess promising fungi for production of pectinases demonstrated that the deuteromycete saprophytic fungus Penicillium griseoroseum is a good producer of pectinolytic enzymes when cultured in medium supplemented with sucrose and yeast extract (Baracat-Pereira et al., 1994; Pereira et al., 2002). A systematic program aiming to improve enzyme production by microorganisms relies on comprehension of the regulation of the genes involved in the overall processes as well-finding alternative, less expensive, sources of energy that promote efficient production of the enzymes of interest. Transformation is a widely employed approach to conduct studies aiming at the comprehension of molecular processes in fungi (see Brakhage and Langfelder, 2002 and Gossen and Bujard, 2002). Among the various selection systems employed in the transformation of fungi, the nitrate reductase system offers several inherent advantages for transformation, such as easy recipient isolation, through its resistance to chlorate, and low background growth after transformation (Daboussi et al., 1989). This is a good system to be used for co-transformation, in cases in which a transforming gene cannot easily be directly selected for. The niaD gene has also been described as a gene trap for the isolation of transposons in fungi (Langin et al., 1995). Green fluorescent protein (GFP) is a 27-kDa protein isolated from the marine jellyfish Aequorea victoria (Prasher et al., 1992). Several studies have already described applications of GFP technology for various purposes. So far, GFP has been used as a reporter for gene expression (Biao Ma and Gold, 2001), for tagging of proteins to monitor their localization within living cells (Borneman et al., 2001) and for monitoring of enzyme secretion (Gordon et al., 2000). The success of GFP as a reporter protein can be attributed to its unique qualities, such as non-requirement of co-factors or substrates for its activity, high stability under different temperature and pH regimes, the possibility of being fused to other proteins without losing its fluorescence property, and the ease with which it is detected in vivo (Lorang et al., 2001). P. griseoroseum is an excellent biological system for studies of molecular genetics of production of industrially important enzymes, such as pectinolytic enzymes, and genetic transformation is a useful tool to investigate these processes. We report the successful co-transformation of P. griseoroseum with the niaD and gfp genes, as well the best parameters for this transformation system. MATERIAL AND METHODS Strains and plasmids The wild-type strain of P. griseoroseum was isolated by Dr. J.J. Muchovej of the Departamento de Fitopatologia, Universidade Federal de Viçosa. The P. griseoroseum PG63 (Nia mutant) was derived from the wild-type strain and was used as the recipient strain in transformation experiments. The plasmid pNPG1 contains the entire nia gene from P. griseoroseum cloned into the KpnI site of the plasmid pBluescript SK II+ (Pereira et al., 2004), and pAN52-1-GFP carries the SGFP-TYG version of GFP under the control of the strong and constitutive Aspergillus nidulans promoter, gpd. Growth and culture conditions The PG63 Nia mutant was cultured on complete medium (Pontecorvo et al., 1953). The wild-type and the transformant strains were cultured on nitrate minimal medium (Pontecorvo et al., 1953). All strains were incubated at 28°C. Co-transformation of Penicillium griseoroseum The transformation procedures were performed according to the basic polyethylene glycol (PEG)-mediated method (Yelton et al., 1984; Ballance and Turner, 1985), with modifications. Protoplasts of the PG63 strain were obtained from mycelia grown on complete medium overlaid with cellophane paper for 24 h at 25°C. About 800 mg mycelia was digested with 15 mg GLUCANEX® enzyme (Novo Nordisk) in 5 ml osmotic stabilizer (0.8 M KCl, 10 mM phosphate buffer, pH 5.8), for 3 h, at 30°C, 80 rpm in orbital shaker. After filtration through cheesecloth, the protoplasts were washed three times in SCT (1 M sorbitol, 50 mM CaCl2 and 100 mM Tris-HCl, pH 7.5), once in 10 ml and twice in 5 ml by centrifugation at 2.3 g, 4°C, for 15 min. The sediment was homogenized in SCT to a final concentration of 108 protoplasts/ml. Plasmidial DNA and 50 µl 60% PEG 6000, previously prepared in SCT, were added to 200 µl of a protoplast suspension containing approximately 107 protoplasts. The preparation was incubated for 20 min on ice before the addition of 500 µl of the same PEG solution. The mixture was kept for 20 min at 25°C and plated on minimal medium. The osmotic stabilizer present in the medium was 0.56 M sucrose. The cultures were incubated for five days at 28°C. Different combined amounts of the plasmids were supplied in each treatment. Screening of co-transformants and fluorescence microscopy The transformants for the gfp gene were screened among the colonies retrieved from the nitrate minimal medium. To determine the co-transformation percentage, 100 colonies from each treatment (Table 1) were randomly selected for epifluorescence analysis. Thin mycelial fragments harvested from each colony were arranged upon a glass slide surface, covered with a coverslip and analyzed under fluorescent light. Epifluorescence images were obtained with an Olympus BX-60 microscope equipped with a 460- to 480-nm excitation filter set, captured with an Olympus (U-CMAD-2) camera and edited with the image analyzer program Image Pro® Plus, version 4.0 (Medial Cybernetics, L.P., 1998). Molecular analysis Genomic DNA from the wild type, PG63 Nia mutant, and five co-transformants (CT8, CT9, CT19, CT20, and CT21) was prepared from fresh mycelium (Specht et al., 1982), digested with the restriction enzyme SmaI, which cuts the plasmid pAN52-1-GFP once, size-separated on 0.8% agarose/TEB gel (Sambroock et al., 1989), transfered onto a nylon membrane (Duralon-UVTM Stratagene) and hybridized at high stringency (65°C/0.2X SSC/0.1% SDS). The probe was an NcoI-BamHI fragment excised from the pAN52-1-GFP plasmid, which contains the entire gfp gene. The probe labelling was performed with the Prime-it® Fluor Fluorescence Labeling Kit and detection was done with the IlluminatorTM Nonradioactive Detection System, both manufactured by Stratagene. All procedures were performed according to the recommendations of the supplier. RESULTS The transformation efficiency increased when pAN52-1-GFP was added to the transformation mixture, when compared to procedures carried out only with the plasmid pNPG1 (Table 1). The highest transformation efficiency (102 transformants/µg of pNPG1) was achieved when equimolar amounts of the transforming and co-transforming vectors were used. Increasing amounts of transforming DNA caused the efficiency of transformation to decrease. The highest co-transformation efficiency (7%) was observed when a ratio of 3:6 pNPG1 and pAN52-1-GFP, respectively, was employed. More than 6 µg of pAN52-1-GFP caused the efficiency of co-transformation to decrease.
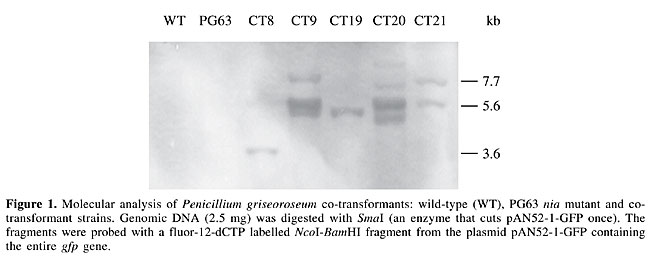
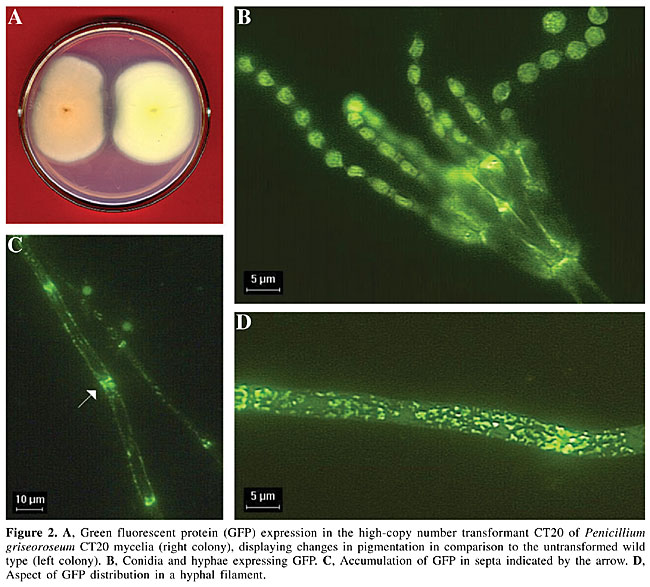
Five co-transformants were selected for hybridization analysis of plasmid integrations. The selected co-transformants had a variable pattern of gfp integration (Figure 1). Some hybridizing bands were intense and showed a typical pattern of tandem integration (Figure 1, CT9 and CT20). The co-transformants CT9 and CT20 also displayed bright green-yellowish mycelial pigmentation (Figure 2A). Since transformants CT8, CT19 and CT21 presented few copies of gfp and did not show alteration in mycelial pigmentation, it is possible that this mycelial aspect is a consequence of a high level of GFP expression in CT9 and CT20 and its accumulation into their hyphae. Epifluorescence microscopy analysis showed that GFP was distributed in the hyphae and conidia (Figure 2B, C and D). In the hyphae, GFP was mainly aggregated in septa (Figure 2C and D).
DISCUSSION Pectinases are involved in the degradation of pectin. They are of considerable importance for the food and textile industries. Processing of beverages (Alkorta et al., 1998) and degumming of plant fibers, such as ramie and linen (Kashyap et al., 2001), are some examples of their industrial importance. Penicillium species are potential producers of pectinases because of their saprophytic life style (Hershenhorn et al., 1990; O’Neill et al., 1991). Previous studies aiming to improve pectinase production by the fungus P. griseoroseum have shown its potential as a starter strain, through enzymatic assays, mainly because of its low cellulase activity, associated with high pectinase activity (Baracat-Pereira et al., 1994; Pereira et al., 2002). We report the co-transformation of P. griseoroseum with the niaD and gfp genes. All treatments where the two plasmids were present showed an increase in the transformation efficiency when compared to the treatment where the niaD gene was used alone. According to Fincham (1989), one possible cause of the increase of the transformation efficiency observed by the utilization of two different kinds of vectors during the transformation is that, if protoplasts are exposed to two different kinds of DNA simultaneously, there is a high probability that a cell that takes up one will also take up the other. A low co-transformation efficiency (4-7%) was observed in all treatments. Co-transformation efficiencies vary and seem to be dependent on the study organism and on experimental conditions. A co-transformation efficiency of 68% was reported for Trichoderma reesei, when equal amounts of two plasmids having different selective markers were used (Pentillä et al., 1987). Punt et al. (1987) analyzed the co-transformation efficiency for Aspergillus nidulans and Aspergillus niger and found 60 and 80% co-transformation efficiency, respectively. The version SGFP-TYG was efficiently expressed in P. griseoroseum. Hybridization analysis revealed transformants carrying single or multiple copies of the gfp gene. Transformants with a high copy number of pAN52-1-GFP showed a bright green yellowish pigmentation in mycelia that was easily noticed without the aid of any special equipment. The observation in the present study that GFP was successfully expressed in P. griseoroseum opens up new possibilities to more thoroughly study the biological processes involved in the production and secretion of pectinases in this organism. ACKNOWLEDGMENTS We thank FAPEMIG and CNPq for providing financial support, Dr. Corinne Clavé, of Institut de Biochimie et de Genetique Cellulaires, Bordeaux, França, for providing the plasmid pAN52-1-GFP and Dr. Silvia Pompolo, of Departamento de Biologia Geral, Universidade Federal de Viçosa, for helping with the fluorescence images. REFERENCES Alkorta, L., Garbisu, C., Llama, M.J. and Serra, J.L. (1998). Industrial application of pectic enzymes: a review. Process Biochem. 33: 21-28. Ballance, D.J. and Turner, G. (1985). Development of a high-frequency transforming vector for Aspergillus nidulans. Gene 36: 321-331. Baracat-Pereira, M.C., Coelho, J.L.C. and Silva, D.O. (1994). Production of pectin lyase by Penicillium griseoroseum cultures on sucrose and yeast extract for degguming of natural fibres. Lett. Appl. Microbiol. 18: 127-129. Biao Ma, M.B.M. and Gold, M.H. (2001). The green fluorescent protein gene functions as a reporter of gene expression in Phanerochaete chrysosporium. Appl. Env. Microbiol. 67: 948-955. Borneman, A.R., Hynes, M.J. and Adrianopoulos, A. (2001). An STE12 homolog from the asexual, dimorphic fungus Penicillium marneffei complements the defect in sexual development of an Aspergillus nidulans steA mutant. Genetics 157: 1003-1014. Brakhage, A.A. and Langfelder, K. (2002). Menacing mold: The molecular biology of Aspergillus fumigatus. Annu. Rev. Microbiol. 56: 433-455. Daboussi, M.J., Djeballi, A., Gerlinger, C., Blaiseau, PL., Bouvier, I., Cassan, M., Lebrun, M.H., Parisot, D. and Brygoo, Y. (1989). Transformation of seven species of filamentous fungi using the nitrate reductase gene of Aspergillus nidulans. Curr. Genet. 15: 453-456. Fincham, J.R.S. (1989). Transformation in fungi. Microbiol. Rev. 53: 148-170. Gordon, C.L., archer, D.B., Jeenes, D.J., Doonan, J.H., Wells, B., Trinci, A.P.J. and Robson, G.D. (2000). A glucoamylase::GFP gene fusion to study protein secretion by individual hyphae of Aspergillus niger. J. Microbiol. Methods 42: 39-48. Gossen, M. and Bujard, H. (2002). Studying gene function in eucaryotes by conditional gene inactivation. Annu. Rev. Genet. 36: 153-173. Hershenhorn, J., Manulis, S. and Barash, L. (1990). Polygalacturonases associated with infection of Valencia orange by Penicillium italicum. Phytopathology 80: 1374-1376. Kashyap, D.R., Vohra, P.K., Chopra, S. and Tewari, R. (2001). Applications of pectinases in the commercial sector: a review. Biores. Technol. 77: 215-227. Kawano, C.Y., Chellegatti, M.A.S.C., Said, S. and Fonseca, M.J.V. (1999). Comparative study of intracellular and extracellular pectinases produced by Penicillium frequentans. Biotechnol. Appl. Biochem. 29: 133-140. Langin, T., Capy, P. and Daboussi, M.J. (1995). The transposable element impala, a fungal member of the Tc1-mariner superfamily. Mol. Gen. Genet. 246: 19-28. Lorang, J.M., Tuori, R.P., Martinez, J.P., Sawyer, T.L., Redman, R.S., Rolling, J.A., Wolpert, T.J., Johnson, K.B., Rodriguez, R.J., Dickman, M.B. and Ciuffetti, L.M. (2001). Green fluorescent protein is lighting up fungal biology (Minireview). Appl. Environ. Microbiol. 67: 1987-1994. O´Neill, T.M., Bagabe, M. and Ann, D.M. (1991). Aspects of biology and control of a stem rot of cucumber caused by Penicillium oxalicum. Plant Pathol. 40: 78-84. Pentillä, M., Nevalainen, H., Rättö, M., Salminen, E. and Knowles, J.K.C. (1987). A versatile transformation system for the cellulolytic filamentous fungus Thrichoderma reesei. Gene 61: 155-164. Pereira, J.F., de Queiroz, M.V., Gomes, E.A., Muro-Abad, J.I. and de Araújo, E.F. (2002). Molecular characterization and evaluation of pectinase and cellulase production of Penicillium spp. Biotechnol. Lett. 24: 831-838. Pereira, J., Queiroz, M.V., Lopes, F.J.F., Rocha, R.B., Daboussi, M.J. and Araújo, E.F. (2004). Characterization, regulation and phylogenetic analyses of the Penicillium griseoroseum nitrate reductase gene and its use as selection marker for homologous transformation. Can. J. Microbiol. (In press). Pontecorvo, G., Roper, J.A., Hemmons, L.M., MacDonald, K.D. and Bufton, A.W. (1953). The genetics of Aspergillus nidulans. Adv. Genet. 5: 141-238. Prasher, D.C., Eckenrod, V.K., Ward, W.W., Prendergast, F.G. and Cormier, M.J. (1992). Primary structure of the Aequorea victoria green fluorescent protein. Gene 111: 229-233. Punt, P.J., Oliver, R.P., Dingemanse, M.A., Pouwels, P.H. and van den Hondel, C.A.M.J.J. (1987). Transformation of Aspergillus based on the hygromycin B resistance marker from E. coli. Gene 56: 117-124. Sambroock, J., Fritsch, E.F. and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA. Specht, C.A., Dirusso, C.C., Novotny, C.P. and Ullrich, R.C. (1982). A method for extracting high molecular weight deoxyribonucleic acid from fungi. Anal. Biochem. 119: 158-163. Yelton, M.M., Hamer, J.E. and Timberlake, W.E. (1984). Transformation of Aspergillus nidulans by using a trpC plasmid. Proc. Nat. Acad. Sci. USA 81: 1470-1474. |
|