
ABSTRACT. Cysteine proteinases (CPs) are synthesized as zymogens and converted to mature proteinase forms by proteolytic cleavage and release of their pro domain peptides. A cDNA encoding a papain-like CP, called hgcp-Iv, was isolated from a Heterodera glycines J2 cDNA library, expressed and utilized to assess the ability of its propeptide to inhibit proteinase in its active form. The hgcp-Iv cDNA sequence encodes a polypeptide of 374 amino acids with the same domain organization as other cathepsin L-like CPs, including a hydrophobic signal sequence and a pro domain region. HGCP-Iv, produced in Escherichia coli as a fusion protein with thioredoxin, degrades the synthetic peptide benzyloxycarbonyl-Phe-Arg-7-amido-4-methylcoumarin and is inhibited by E-64, a substrate and inhibitor commonly used for functional characterization of CPs. Recombinant propeptides of HGCP-Iv, expressed in E. coli, presented high inhibitory activity in vitro towards its cognate enzyme and proteinase activity of Meloidogyne incognita females, suggesting its usefulness in inhibiting nematode CPs in biological systems. Cysteine proteinases from other species produced no noticeable activity. Key words: Cysteine proteinase, Pro domain peptide, Expression, Inhibitory activity, Nematode, Heterodera glycines INTRODUCTION Sedentary endoparasitic root-knot (Meloidogyne spp.) and cyst (Globodera spp. and Heterodera spp.) nematodes are the greatest worldwide cause of economic damage in several crop plants. Common pesticide control methods may not be effective in preventing nematode infection; consequently, new control strategies are essential for developing countries to achieve sustainable agricultural production. One promising and powerful strategy to control these endoparasites is engineered plants with anti-nutritional factors. As key enzymes in parasite metabolism and host-pathogen interaction, proteinases are potential targets for nematode control by plants. Different proteinase classes (cysteine, serine, aspartic, and metalloproteinases) are present in parasitic nematodes. Many of which are thought to be involved in invading or fending off invasions of host tissues, as well as parasite nutrition (Lilley et al., 1996), and interfering with these proteinases could help protect plants by disrupting parasite digestion (Lilley et al., 1999). High cysteine proteinase (CP) activity in the intestine of the plant-parasite nematodes Globodera pallida (Koritas and Atkinson, 1994) and Heterodera glycines (Lilley et al., 1996) indicated that this enzyme class plays an important role in the digestive feeding stages of nematodes. Tomato roots expressing the CP inhibitors, oryzacystatin-I (Oc-I) and a variant form Oc-IDD86, retard growth and development of the potato cyst-nematode G. pallida (Urwin et al., 1995). Two cDNAs encoding CPs have been isolated and characterized in H. glycines females. Both codify the predicted proteins with a short-secretion signal sequence, long propeptides and mature proteins with 219-amino acid residues (Urwin et al., 1997a); however, comparison of these two sequences against the database predicted one cDNA (hgcp-I) to encode a cathepsin L-like proteinase and the other (hgcp-II) to encode a cathepsin S-like enzyme. High activity of cathepsin L-like cysteine proteinase found in the intestine of H. glycines feeding females (Lilley et al., 1996; Urwin et al., 1997a) indicates that this enzyme class is a good target for disrupting feeding. Cathepsin-like proteins are synthesized as inactive proenzymes with N-terminal pro domain regions (Yamamoto et al., 2002). The following aspects of the role of pro domain peptides have been described: protein folding (Shi et al., 2001; Schilling et al., 2001), protein transportation to the correct intracellular destination (Song and Fricker, 1997; Tang et al., 2002, 2003), and inhibitory action against corresponding enzymes (Carmona et al., 1996; Chagas et al., 1996; Lalmanach et al., 1998; Visal et al., 1998; Roche et al., 1999). The inhibitory action of proregion peptides in both subfamilies of procathepsin L and B occurs by obstructing substrate access to the enzyme active site, thus preventing enzyme proteolytic activities (Cygler and Mort, 1997). Proregions and the substrate follow the groove in opposing directions, causing the peptide bond to be inappropriately positioned for hydrolysis (Podobnik et al., 1997). Human cathepsin L propeptide is a potent inhibitor of the mature enzyme and pH-dependent, indicating that the inhibition mechanism involves more than one step as well as reflecting the ionization state of residues on the inhibitor prior to binding to the enzyme (Carmona et al., 1996). The pro domain peptide of papaya proteinase IV inhibits Colorado potato beetle digestive CPs and other papaya proteinases, including papain, suggesting that it could be a general inhibitor of papain-like CPs (Visal et al., 1998). We explored the use of pro domain peptides as a strategy to control plant sedentary nematodes. We described cloning and expression of the proregion and mature enzyme of a new variant form of H. glycines cysteine proteinase I (hgcp-Iv), in Escherichia coli in order to access the inhibitory potential of the propeptide towards its cognate enzyme. The activity of a recombinant proregion was also determined towards crude soluble extract of J2 Meloidogyne incognita and midgut crude extracts of some insect pests to evaluate its selectivity and/or specificity. MATERIAL AND METHODS Isolation and sequence analysis of Heterodera glycines cysteine proteinase cDNA A 32P-labeled in vitro transcribed 141-bp homologous probe was used to screen the H. glycines J2 cDNA library in Uni-ZAP (Stratagene) (Smant et al., 1998). The probe was amplified by PCR using degenerate primers based on conserved CGSCW and GCNGG segments found in a number of CPs and the H. glycines J2 cDNA library. Successive rounds of screening purified plaques yielding duplicate positive signals until all plaques yielded positive signals. The cDNA inserts were rescued in pBluescript plasmid using the Exassit/SOLR system (Stratagene). Recombinant clones were sequenced in both strands in an automated DNA sequencer. Computer analysis of DNA and derived amino acid sequences were performed using the GCG package (Genetics Computer Group, Inc.), bioinformatics resources of the NCBI homepage (http://www.ncbi.nlm.nih.gov) and the EBI website (http://www.ebi.ac.uk/). Expressed sequence tags (ESTs) were submitted to BLASTx analysis (NCBI) and grouped according to identity. Related ESTs were aligned by CLUSTALW (http://www.ebi.ac.uk/clustalw/). Translations were performed using ExPASy Proteomics tools from their homepage (http://us.expasy.org/). Peptide signal was predicted with SignalP software (http://www.cbs.dtu.dk/), and pro and mature regions were determined by sequence comparisons. HGCP and PROHGCP expression and refolding in Escherichia coli Sequences encoding hgcp-Iv and prohgcp were amplified by PCR and subcloned into pET102D TOPO® (Invitrogen). Recombinant proteins were fused with thioredoxin at the N-terminal and a 6X His tag at the C-terminal. Recombinant HGCP-Iv and PROHGCP (strain BL21 DE3) were induced with 0.5 mM isopropyl-1-thiol-b-D-galactopyranoside (IPTG) for 3 h. Solubility of the recombinant proteins was analyzed under different temperatures and salt concentrations. E. coli pellet cells were incubated for 1 h/25°C in buffer A (8 M urea, 0.1 M NaH2PO4, 0.01 M Tris-HCl) at pH 8.0 under continuous shaking. Lysate was centrifuged at 10,000 g for 20 min. The supernatant was loaded into an affinity Ni-NTA (Qiagen®) column. Unbound proteins were removed with 5 washes in buffer A at pH 6.3. Recombinant protein was eluted from the resin using buffer A at pH 4.5, dialyzed against buffer 0.1 M Tris-HCl, pH 8.0, to avoid precipitation of the recombinant proteins, followed by dialysis in equilibration buffer 0.5 M Tris-HCl, 10 mM CaCl2, 1% Tween 20, and pH 8.0 for digestion. Purified recombinant proteins were then digested with enterokinase for 40 h to remove the N-terminal leader. Digested HGCP-Iv was refolded by slow drop-wise dilution ( Proteolytic activity and proteinase inhibitory assays Instar larvae midguts of Acanthoscelides obtectus (bean weevil; Coleoptera) and Spodoptera frugiperda (fall armyworm; Lepidoptera) were dissected in ice-cold 0.1 M Tris-HCl, pH 8.0. Freshly dissected guts were homogenized and centrifuged at 4,000 g for 20 min at 4°C to remove gut walls and cellular debris. The supernatant was used to measure CP proteolytic activity and to analyze inhibitory activity of PROHGCP against these proteinases. A crude soluble extract of female nematode M. incognita was homogenized in buffer 0.1 M Tris-HCl, pH 8.0, and centrifuged as previously described. The supernatant ( RESULTS Initial amplification of H. glycines CP genes was performed by PCR using degenerate primers based on conserved segments found in CP sequences from several organisms and an H. glycines J2 cDNA library as a template. An amplified 141-bp segment encoding a fragment homologous to other CP genes was used as a probe to screen approximately 100,000 plaques from the J2 cDNA library, resulting in 50 reactive clones. Six were chosen for sequence analysis. All clones were found to encode the same gene, resulting in a 1,338-bp cDNA containing a 1,122-bp open reading frame that encodes a 374-amino acid protein similar to other CPs. Comparison with other nematode proteinase genes revealed that the amplified sequence is 99% identical at the amino acid sequence to HGCP-I, a CP previously isolated from H. glycines females by Urwin et al. (1997a) (Figure 1). Only 10 nucleotide point substitutions were found between the two sequences, six of which are located within the coding region. Three substitutions in the mature protein resulted in an amino acid change, and two substitutions are conservative (Figure 1). This variant form is herein termed hgcp-Iv. 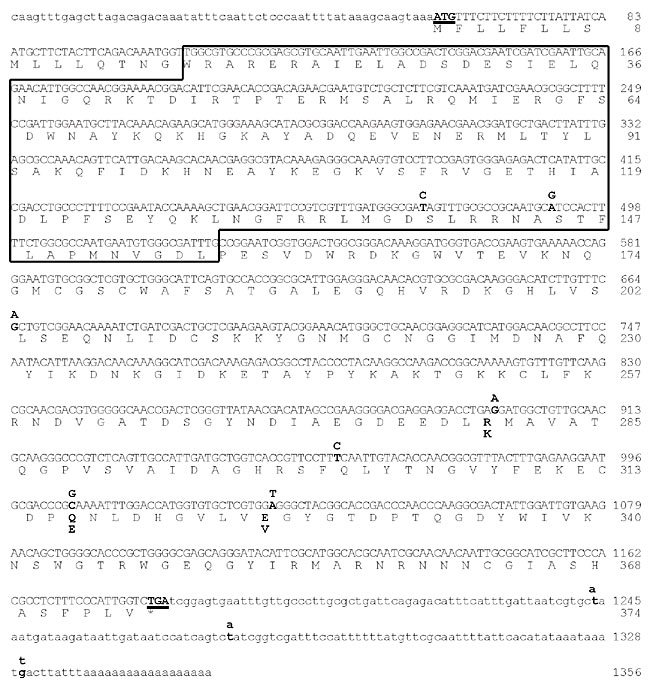
Figure 1. Nucleotide and predicted amino acid sequences of hgcp-Iv. Coding sequence is shown in upper case letters and 3´ and 5´ untranslated regions in lower case letters. Start codon is shown in bold and underlined. Stop codon is shown in bold, underlined and with an asterisk. The proregion is boxed. Nucleotide differences to hgcp-I are shown above the sequence and amino acid differences are show below the sequence, both in bold.
The variant is similar to HGCP-I in that both contain a 16-residue predicted signal peptide followed by a 139-amino acid proregion sequence and a mature 219-amino acid protein. Predicted molecular weights are approximately 16 kDa for HGCP-I and 24 kDa for HGCP-Iv. The proregion was identified through databank searches for sequence homology and comparison with other CP genes. M. incognita CP gene was highly similar to HGCP-Iv (65.5% identity at the amino acid level) (Figure 2). Sixteen ESTs, encoding M. incognita CPs, were found to correspond to three distinct genes. One is represented by nine ESTs and has a mean identity of 64% and a 1,183 bp overlap with hgcp-Iv. The other two varied more widely and have not yet been investigated. Sequence analysis revealed that the nine ESTs similar to hgcp-Iv correspond to a cDNA of 1,176 bp that encodes a proregion of 141-amino acid residues and a mature region of 221 residues. Identities of HGCP-Iv proregion and mature protein with M. incognita enzyme were 55 and 76%, respectively. Distinct from other CPs, HGCP-Iv and M. incognita sequences present a 38-residue N-terminal extension with 29.3% identity. 
Figure 2. Sequence alignment of cathepsin L-like pro-mature regions from several species. Pre-regions were detected by SignalP, but omitted in the alignment. Sequences are from Heterodera glycines HGCP-Iv, Meloidogyne incognita (contig from ESTs AW783136, AW827872, AW828007, AW828202, AW828905, AW829383, AW870910, CF803069, CF802613), Dictyocaulus viviparus (AAK77918), Haemonchus contortus (AAL14224), Caenorhabditis elegans (NP_507199), Homo sapiens (NP_001903), A. obtectus (AAQ22984) and papain from Carica papaya (P05994). Arrowheads mark amino acid residues of the catalytic triad, and a line divides pro- and mature regions. DNA sequence of hgcp-Iv has been submitted to GenBank under accession number AY554271.
In general, identity between HGCP-Iv mature enzyme sequence and other CP sequences was greater than that between CP proregions. For example, human and HGCP-Iv mature enzyme sequences present 61% identity, while their proregions have only 21% identity. This value is near pro domain sequence identity between HGCP-Iv and HGCP-II, an H. glycines cathepsin-S (Urwin et al., 1997a). The HGCP-Iv proregion presented a mean identity of 40.4% with other nematode proregions, while sequence similarity with papain and a human cathepsin L was much lower, with a mean identity of 20.7%. Among the enzymes compared in Figure 2, a CP sequence from the coleopteran pest A. obtectus was the most different from HGCP-IV with 15.8% identity at the proregion sequence and 46.4% identity in the mature enzyme sequence. Pro domain peptide and mature CP sequences were expressed in E. coli in order to study the effect of the HGCP-Iv pro domain peptide on proteolytic activity of its cognate enzyme and CP activities of other agricultural pests. A schematic representation of the final vectors showing both constructions is presented in Figure 3. Fusion with thioredoxin was chosen in both constructions to increase solubility, stability and to avoid degradation of the recombinant proteins. SDS-PAGE analysis of total protein extracts from induced bacterial clones harboring the constructs revealed a band of approximately 55 kDa for the mature HGCP-Iv protein (Figure 4A, lane 3) and 31 kDa for its propeptide (Figure 4B, lane 3). Sizes were as expected for each recombinant protein and peptide. Neither band was present in extracts from uninduced cells (Figure 4A and B, lanes 2). Although several changes in salt and IPTG concentrations, time and temperature of induction were examined, formation of inclusion bodies was not avoided, and the recombinant proteins were always located in the insoluble cellular fraction. Inclusion bodies were solubilized in 8 M urea, and the recombinant proteins were purified by affinity chromatography in Ni-NTA columns (Figure 5). 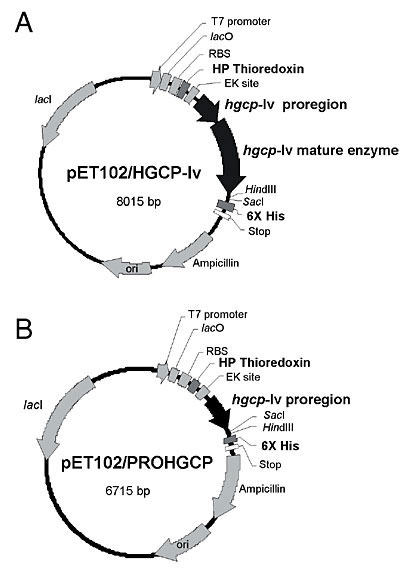
Figure 3. Constructions of hgcp-Iv and prohgcp vectors for expression in Escherichia coli using the pET system. Both sequences encoding the mature form and the propeptide region were amplified by PCR and cloned in frame with thioredoxin with a His-tag tail at the 3’ region. A, Mature form. B, Propeptide.
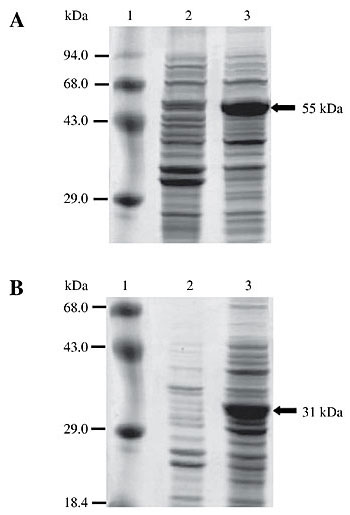
Figure 4. Expression of recombinant mature and propeptide HGCP-Iv in an Escherichia coli system. Expression of recombinant proteins was induced with IPTG for 3 h at 37°C. Total protein extracts were analyzed by 12% SDS-PAGE and stained with Coomassie blue. A, Mature form. B, Propeptide. Lane 1, Molecular weight markers; lane 2, non-induced extracts, and lane 3, induced extracts. Recombinant proteins are indicated with an arrow.
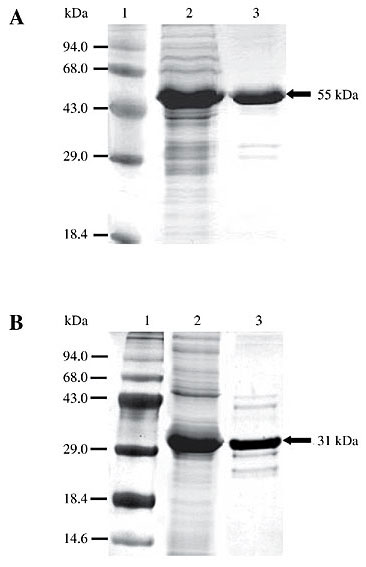
Figure 5. Purification of mature form and propeptide of recombinant HGCP-Iv. Insoluble protein extracts from both constructions were solubilized in 8 M urea, and recombinant proteins were purified in Ni-NTA columns. A, Mature form. B, Propeptide. Lane 1, Molecular weight markers; lane 2, total extracts, and lane 3, purified proteins. Recombinant proteins are indicated with an arrow.
To eliminate any possibility of thioredoxin influence on propeptide activity and the mature HGCP-Iv form, recombinant fusion proteins were digested with enterokinase, and digestions were monitored by SDS-PAGE (data not shown). Afterwards, recombinant HGCP-Iv mature protein was inactive, and recovery of proteolytic activity was achieved by slow serial dilution. Although several buffers were evaluated, proteolytic activity was only detected when sodium phosphate buffer (0.1 M Na2PO4, 3 mM DTT, 2 mM EDTA, pH 6.0) was utilized for refolding. PROHGCP peptide inhibitory activity towards mature cognate enzyme was determined by in vitro fluorimetric assays using Z-Phe-Arg-AMC as substrate (Figure 6). Proteolytic activity of HGCP-Iv recombinant protein was completely inhibited by the PROHGCP recombinant peptide and E-64, a specific synthetic inhibitor of CP-like enzymes. Previous incubation of PROHGCP peptide with antiserum raised against the propeptide completely abolished its inhibitory activity against the pro-enzyme. 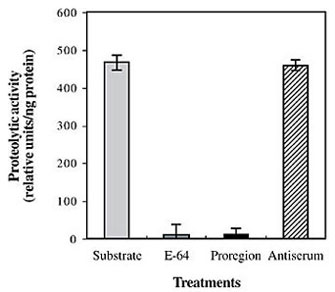
Figure 6. Recombinant PROHGCP inhibitory activity against recombinant HGCP-Iv. Fluorimetric assays were performed in a DyNA Quant 500 fluorescence reader (Pharmacia-Biotech), using Z-Phe-Arg-AMC as substrate (10 µM). Inhibitors were pre-incubated with the enzymes before measuring proteolytic activity. Assays were performed in triplicate.
Specificity of the pro domain peptide on inhibitory activity was assayed against crude soluble extracts from M. incognita (Nematode), A. obtectus (Coleoptera) and S. frugiperda (Lepidoptera). Since the Z-Phe-Arg-AMC substrate can also be cleaved by trypsin, specific inhibitors were used to determine the level of activity corresponding to CP activity in M. incognita female extracts. Incubation with E-64 inhibitor reduced 90% of the proteolytic activity while incubation with PMSF inhibited only 12% of the activity (data not shown), confirming that most of the activity measured in M. incognita extracts was due to CP enzymes. PROHGCP displays a strong inhibition towards CP nematode activity, which can be reverted by incubating with the antiserum raised against the proregion, depicting the specificity of the inhibition (Figure 7). On the other hand, PROHGCP had no effect on the proteolytic activity of either insect. 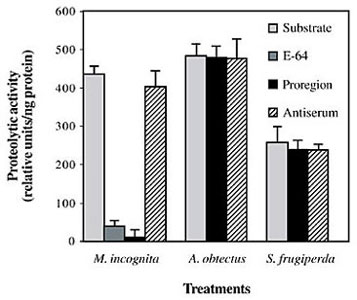
Figure 7. Recombinant PROHGCP inhibitory activity against crude soluble extracts. Fluorimetric assays were performed in a DyNA Quant 500 fluorescence reader (Pharmacia-Biotech), using Z-Phe-Arg-AMC as substrate (10 µM). Inhibitors were pre-incubated with the enzymes before measuring proteolytic activity. Assays were performed in triplicate.
DISCUSSION To protect cells from uncontrolled degradation, almost all proteinases are synthesized as inactive precursors, whose stability is maintained by the propeptides that interact with the surface of mature enzymes at various points. The inactivation mechanism of both enzymes is similar in mammalian B and L cathepsins and involves part of the proregion entering the substrate binding cleft in a reverse (C-N) orientation to natural substrate blocking access to the active site (Roche et al., 1999). Several studies demonstrated that free propeptides are potent inhibitors of their corresponding mature enzymes (Chagas et al., 1996; Lalmanach et al., 1998; Visal et al., 1998; Yamamoto et al., 2002). In the present study, we assessed the potential of PROHGCP to inhibit nematode proteinases. Isolation of a cDNA encoding the preprocathepsin L variant of H. glycines (hgcp-Iv) and its functional expression in E. coli, purification and refolding in vitro were described. The recombinant propeptide was purified and assayed towards the cognate enzyme and other nematode and plant insect pest CPs to evaluate its selectivity and/or specificity. Expression level of recombinant proteins was high, but both recombinant proteins were expressed as inactive insoluble proteins. Recovery of activity was only achieved by slow serial dilution in sodium phosphate buffer. In accordance with the literature (Chagas et al., 1996; Lalmanach et al., 1998; Visal et al., 1998; Roche et al., 1999; Yamamoto et al., 2002), data from the present study showed that PROHGCP is a potent inhibitor of the cognate enzyme, indicating that the HGCP-Iv proregion could be used as a novel inhibitor to disrupt digestion of H. glycines. Thus far the only transgenic approach to demonstrate clear positive results in controlling plant parasitic nematodes is based on blocking nematode proteinases (Davis et al., 2000). CPs represent predominant proteinase activity in plant parasitic nematodes (Lilley et al., 1999) located in the intestine of H. glycines (Lilley et al., 1996) and G. pallida (Koritas and Atkinson, 1994), and also detected in M. hapla, M. javanica and M. incognita (Michaud et al., 1996). The CP inhibitor Oc-IDD86, a modified rice cystatin expressed in transgenic Arabidopsis thaliana, can suppress growth and fecundity of both cyst and root-knot nematodes by disrupting digestive proteinases (Urwin et al., 1997b). Consequently, by targeting a common plant-nematode process, an anti-feeding strategy could control a broad range of parasite species within three genera (Heterodera, Globodera and Meloidogyne). To access the capability of a peptide to inhibit activity across species, we tested the recombinant PROHGCP-Iv peptide against the crude soluble extract of M. incognita females. Similar to the inhibitory activity over the cognate enzyme, PROHGCP was highly effective in inhibiting CP activity in M. incognita females. However, a more detailed analysis of this data was hampered by the fact that there are no complete reports on cloning and characterization of CP genes in M. incognita. Consequently, a homology search of M. incognita ESTs in databanks was performed to analyze the inhibition assay data in more detail. Nine ESTs were found that corresponded to the same gene with 65.5% identity with HGCP-I at the amino acid sequence level. Indeed, among all sequences compared, M. incognita CP had the highest identity with HGCP-Iv. Furthermore, the relative abundance of ESTs encoding this gene suggests that it corresponds to a major part of CP activity in M. incognita females. The high identity between H. glycines and M. incognita sequences and the predominance of this CP in M. incognita may explain the in vitro inhibitory assays. Since PROHGCP was highly effective in inhibiting CP activity in crude extracts of M. incognita females, we believe that this propeptide will certainly inhibit other CPs homologous to HGCP-Iv in virtually different endoparasitic nematode species. As for current biosafety concerns, foreign toxic proteins expressed in transgenic plants should present no adverse effects in humans, other organisms or even in the transformed plant itself. Our data show that the recombinant propeptide of HGCP-Iv has no effect on CP activity of the two insects tested, coleopteran and lepidopteran. The low level of amino acid identity between HGCP-Iv and papain proregions with human CP indicates that it is unlikely that PROHGCP will have any significant inhibitory effect on these two enzymes. Another advantage of propeptides over other potential anti-nematode effectors, such as lectins, is their small size, which can help in the oral uptake of macromolecules. In H. schachtii, the limit for globular proteins is between 11.2 and 28 kDa (Urwin et al., 1997c). Due to their small size, propeptides would have no such restrictions. In summary, we explored the use of propeptides as an anti-nematode effector for the first time; however, it is still necessary to explore the potential of propeptides to control plant parasitic nematodes in vivo. Molecular engineering of proregions to achieve greater selectivity/specificity, use of more than one effector, and/or use of tissue specific promoters are necessary to produce effective nematode-resistant transgenic plants. We are currently expressing the HGCP-Iv propeptide in transformed soybean roots to analyze its effect on infection, development and reproduction of H. glycines. ACKNOWLEDGMENTS The authors thank Eric L. Davis, of North Carolina State University, for the H. glycines J2 cDNA library. Research supported by grants from the Brazilian government, Embrapa Genetic Resources and Biotechnology, CNPq and CAPES. REFERENCES Carmona, E., Dufour, E., Plouffe, C., Takebe, S., Mason, P., Mort, J.S. and Ménard, R. (1996). Potency and selectivity of the cathepsin L propeptide as an inhibitor of cysteine proteases. Biochemistry 35: 8149-8157. Chagas, J.R., De Martino, M.F., Gauthier, F. and Lalmanach, G. (1996). Inhibition of cathepsin B by its propeptide: use of overlapping peptides to identify a critical segment. FEBS Lett. 392: 233-236. Cygler, M. and Mort, J.S. (1997). Proregion structure of members of the papain superfamily. Mode of inhibition of enzymatic activity. Biochimie 79: 645-652. Davis, E.L., Hussey, R.S. and Baum T.J. (2000). Nematode parasitism genes. Annu. Rev. Phytopathol. 38: 365-396. Grossi-de-Sá, M.F., Martins de Sá, C., Harper, F., Coux, O., Akhayat, O., Pal, J.K., Florentin, Y. and Scherrer, K. (1988). Cytolocalization of prosomes as a function of differentiation. J. Cell. Sci. 89 (Pt 2): 151-165. Koritas, V.M. and Atkinson, H.J. (1994). Proteinases of females of the phytoparasite Globodera pallida (potato cyst nematode). Parasitology 109: 357-365. Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the fead of bacteriophage T4. Nature 227: 680-685. Lalmanach, G., Lecaille, F., Chagas, J.R., Authié, E., Scharfstein, J., Juliano, M.A. and Gauthier, F. (1998). Inhibition of trypanosomal cysteine proteinases by their propeptides. J. Biol. Chem. 273: 25112-25116. Lilley, C.J., Urwin, P.E., McPherson, M.J. and Atkinson, H.J. (1996). Characterization of intestinally active proteinases of cyst-nematodes. Parasitology 113: 415-424. Lilley, C.J., Devlin, P., Urwin, P.E. and Atkinson, H.J. (1999). Parasitic nematodes, proteinases and transgenic plants. Parasitol. Today 15: 414-417. Michaud, D., Cantin, L., Bonade-Bottino, M., Jouanin, L. and Vrain, T.C. (1996). Identification of stable plant cystatin/nematode proteinase complexes using mildly denaturation gelatin/polyacrylamide gel electrophoresis. Electrophoresis 17: 1373-1379. Podobnik, M., Kuhelj, R., Turk, V. and Turk, D. (1997). Crystal structure of the wild-type human procathepsin B at 2.5 Å resolution reveals the native active site of a papain-like cysteine protease zymogen. J. Mol. Biol. 271: 774-788. Roche, L., Tort, J. and Dalton, J.P. (1999). The propeptide of Fasciola hepatica cathepsin L is a potent and selective inhibitor of the mature enzyme. Mol. Biochem. Parasitol. 98: 271-277. Schilling, K., Pietschmann, S., Fehn, M., Wenz, I. and Wiederanders, B. (2001). Folding incompetence of cathepsin L-like cysteine proteases may be compensated by the highly conserved, domain-building N-terminal extension of the proregion. Biol. Chem. 382: 859-865. Shi, X.P., Chen, E., Yin, K.C., Na, S., Garsky, V.M., Lai, M.T., Li, Y.M., Platchek, M., Register, B., Sardana, M.K., Tang, M.J., Thiebeau, J., Wood, T., Shafer, J.A. and Gardel, S.J. (2001). The pro domain of b-secretase does not confer strict zymogen-like properties but does assist proper folding of the proteinase domain. J. Biol. Chem. 276: 10366-10373. Smant, G., Stokkermans, J.P.W.G., Yan, Y., Bôer, J.M., Baum, T.J., Wang, X., Hussey, R.S., Gommers, F.J., Henrissat, B., Davis, E.L., Helder, J., Schots, A. and Bakker, J. (1998). Endogenous cellulases in animals: Isolation of 1,4-endoglucanase genes from two species of plant-parasitic cyst nematodes. Proc. Natl. Acad. Sci. USA 95: 4906-4911. Song, L. and Fricker, L.D. (1997). The pro region is not required for the expression or intracellular routeing of carboxypeptidase E. Biochem. J. 323: 265-271. Tang, B., Nirasawa, S., Kitaoka, M. and Hayashi, K. (2002). The role of the N-terminal propeptide of the pro-aminopeptidase processing protease: refolding, processing, and enzyme inhibition. Biochem. Biophys. Res. Commun. 296: 78-84. Tang, B., Nirasawa, S., Kitaoka, M., Marie-Claire, C. and Hayashi, K. (2003). General function of N-terminal propeptide on assisting protein folding and inhibiting catalytic activity based on observations with a chimeric thermolysin-like proteases. Biochem. Biophys. Res. Commun. 301: 1093-1098. Urwin, P.E., Atkinson, H.J., Waller, D.A. and McPherson, M.J. (1995). Engineered oryzacystatin-I expressed in transgenic hairy roots confers resistance to Globodera pallida. Plant J. 8: 121-131. Urwin, P.E., Lilley, C.J., McPherson, M.J. and Atkinson, H.J. (1997a). Characterization of two cDNA encoding cysteine proteinases from the soybean cyst nematode Heterodera glycines. Parasitology 114: 605-613. Urwin, P.E., Lilley, C.J., McPherson, M.J. and Atkinson, H.J. (1997b). Resistance to both cyst and root-knot nematodes conferred by transgenic Arabidopsis expressing a modified plant cystatin. Plant J. 12: 455-461. Urwin, P.E., Moller, S.G., Lilley, C.J., McPherson, M.J. and Atkinson, H.J. (1997c). Continual green-fluorescent protein monitoring of Cauliflower Mosaic Virus 35S promoter activity in nematode-induced feeding cells in Arabidopsis thaliana. Mol. Plant-Microbe Interact. 10: 394-400. Visal, S., Taylor, M.A.J. and Michaud, D. (1998). The proregion of papaya proteinase IV inhibits Colorado potato beetle digestive cysteine proteinases. FEBS Lett. 434: 401-405. Yamamoto, Y., Kurata, M., Watabe, S., Murakami, R. and Takahashi, S.Y. (2002). Novel cysteine proteinase inhibitors homologous to the proregions of cysteine proteinases. Curr. Protein Peptide Sci. 3: 231-238. |
|