ABSTRACT. Hygienic behavior is a desirable trait in honey bees (Apis mellifera L.), as hygienic bees quickly remove diseased brood, interrupting the infectious cycle. Hygienic lines of honey bees appear to be more sensitive to the odors of dead and diseased honey bee brood, and Africanized honey bees are generally more hygienic than are European honey bees. We compared the number of sensilla placodea, antennal sensory structures involved in the perception of odor, in 10 bees from each of six hygienic and four non-hygienic colonies of Africanized honey bees. The sensilla placodea of three of the terminal segments (flagellomeres) of the right antenna of each bee were counted with a scanning electron microscope. There were no significant differences in the mean numbers of sensilla placodea between the hygienic and non-hygienic bees, though the variance was higher in the hygienic group. Flagellomere 4 had significantly more sensilla placodea than flagellomeres 6 and 8. However, there was no significant difference between the other two flagellomeres. As hygienic bees are capable of identifying dead, injured, or infested brood inside a capped brood cell, sensilla placodea probably have an important role in enabling worker bees to sense sick brood. However, we did not find greater numbers of this sensory structure in the antennae of hygienic, compared to non-hygienic Africanized honey bees. Key words: Hygienic behavior, Honey bee, Africanized honey bee, Antennae, Flagellomere, Plate organs, Sensilla placodea INTRODUCTION Some worker bees (Apis mellifera) are capable of recognizing and removing diseased, damaged, or dead brood in capped brood cells. This uncapping and removal behavior is termed hygienic behavior (Rothenbuhler, 1964a). Despite the unquestionable relevance of the work of the genetic mechanisms studied by Rothenbuhler, it is not clear which mechanisms are used by the bees to identify and recognize the cells that contain dead brood, nor do we know the stimuli involved in this process. It has been demonstrated that this behavior is controlled by two pairs of recessive genes, u (uncapper) and r (remover) (Rothenbuhler, 1964b). Moritz (1998) made a reevaluation of the two-locus model for hygienic behavior in honey bees and proposed a three-locus model, but did test his hypothesis experimentally. Based on experimental work, Gramacho (1999) proposed that the two-locus model should be amplified to three pairs of genes, two pairs of which (u1 and u2) would be responsible for initially making a hole in the cell capping and uncapping, respectively, and one pair for the phase of removal (r) of the brood. Hygienic behavior seems to be a generalized response of the bees towards pathogens and parasites in the colony. Many cues are probably used by honey bees to identify if the larvae or pupae inside a cell are healthy, sick, damaged or infested with mites. The sensory structures in the antennae are likely used by nurse bees to distinguish healthy from “abnormal” brood. The smell of mites inside the cell with the brood, for instance the mite Varroa destructor (Anderson and Trueman, 2000), may stimulate the bees to take action and remove infested brood (Rosenkranz et al., 1993; Boecking and Drescher, 1994; Aumeier, 1995; Aumeier et al., 1996; Sasagawa et al., 1999). Africanized honey bees have been found to be more resistant towards V. destructor than European honey bees (Moretto et al., 1991; De Jong and Gonçalves, 1998; Guerra Jr. et al., 2000). The levels of infection by this mite vary from one region to another, as well as with climate and bee race (De Jong et al., 1984; Kulincevic et al., 1988; Moretto et al., 1991). Gramacho (1999) found differences in hygienic behavior between Africanized honey bees and Carniolan bees. It is also known that worker bees are able to identify and uncap cells infested by V. destructor, removing the infested brood (Peng et al., 1987; Boecking and Drescher, 1992, 1994; Spivak, 1996; Corrêa-Marques and De Jong, 1998), which is hygienic behavior. However, not much is known about the mechanisms that allow the worker bees to recognize brood infested by the mites before they remove them. Factors or stimuli that initiate the hygienic or cleaning behaviors may, initially, stimulate the process of recognition of the dead, sick, or infested brood by the hygienic bees. These factors could include: a) physical: vibrations provoked by the brood or by the mites inside the cell that are recognized by the hygienic workers (Schultz-Langner, 1960), temperature differences between dead and live brood (Gramacho et al., 1998); b) chemicals: volatile substances released by the dead, sick, or damaged brood (e.g., substances in decomposition) or even a lack of production of specific substances that act as “chemical signals” to activate hygienic behavior (Titera and Kokoris, 1994; Spivak and Downey, 1998 ), and c) an interaction between chemical and physical factors, which may also act together to initiate or inhibit hygienic behavior. The behavior of the bees is influenced by external stimuli that can be detected by sensory organs (Warnke, 1976). One of these organs, the antenna, is highly complex, with a series of sensory components, including the plate organs or sensilla placodea, which are used for the perception of odors (McIndoo, 1914; Frisch, 1921; Kaissling and Renner, 1968), sensilla trichodea and sensilla basiconica, which are touch (Frisch, 1921), and chemical receptors, respectively (Snodgrass, 1935, 1956); the sensilla ampullacea and sensilla coeloconica, which are hygroreceptors (Kuwabara and Takeda, 1956) and the sensilla campaniformia, which allow the perception of temperature, CO2, and humidity (Dietz and Humphreys, 1971). Although some researchers have found correlations between the number of sensory structures and some types of behavior, such as defensive behavior (Stort, 1979; Stort and Rebustini, 1998), there have been no investigations of antennal sensory structures and hygienic behavior of honey bees. We examined the number of sensilla placodea in the terminal segments of the antennae of hygienic and non-hygienic Africanized honey bees. MATERIAL AND METHODS Measurement of hygienic behavior The pin-killed brood assay, developed by Newton and Ostasiewski Jr. (1986) and modified by Gramacho and Gonçalves (1994), was used to measure hygienic behavior. This assay consists of piercing the sealed worker brood of the same age (corresponding to the pink eye pupal phase), using a number 1 entomological pin. A test comb with capped brood cells was removed from each colony; 100 brood cells in one area of the comb were perforated, and another 100 cells in the same comb were used as controls. After perforating the brood, the combs were returned to the colonies. After 24 h, the test combs were taken to the laboratory to count the cells that had been cleaned by the bees. The degree of hygienic behavior (HB) was determined as a percentage of cleaned cells 24 h after piercing the brood:
where, EC (24 h) = number of empty cells 24 h after perforation; EC (0 h) = number of empty cells before perforation; CC (0 h) = number of capped cells before perforation, and Z = control correction factor
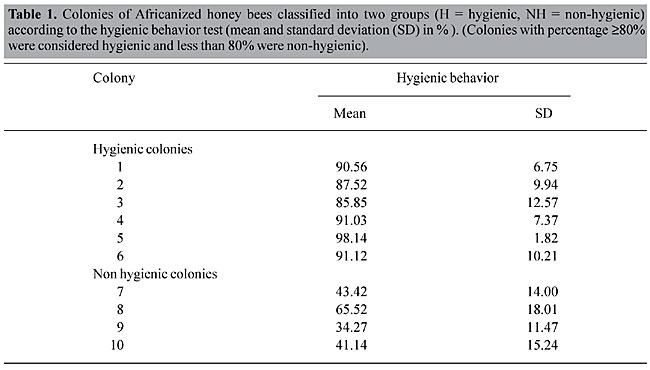
where, A = number of capped cells in the control before placing the comb into the colony for the cleaning test; Y = number of new empty cells, calculated as Y = C – B; C = number of empty cells after the cleaning test, and B = number of empty cells before the cleaning test. Twenty colonies of wild-type Africanized honey bees Apis mellifera L., in an apiary in Ribeirão Preto, SP, were tested for their hygienic behavior. Each of the colonies was tested five times, during a 10-15-day period. A colony was considered hygienic if it removed a mean of 80% or more of the dead brood. Six hygienic and four non-hygienic colonies were selected for the study of sensory structures (Table 1).
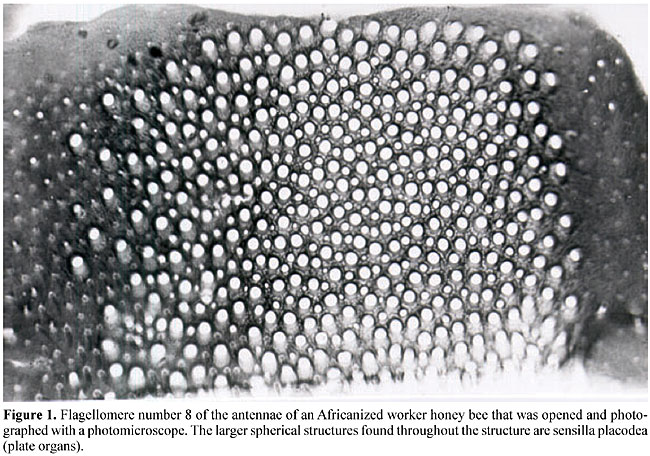
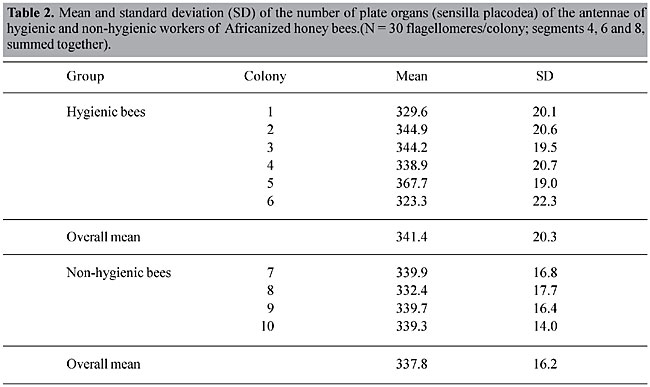
Counting the number of sensilla placodea Ten bees were obtained from each of the colonies and killed by asphyxiation with ether, fixed with Dietrich’s. Subsequently, the right antennae were withdrawn and three segments of the flagellum, or flagellomeres, classified from the base of the antennae as numbers 4, 6 and 8, were separated. Each antennal segment (Figures 1 and 2) was opened longitudinally with two entomological pins and stretched on a histological glass slide, sealed with Canada balsam, and covered with a cover slip. They were photographed with a Zeiss II photo microscope. The film was placed in slide mounts and projected onto a screen, where the plate organs were counted (Stort, 1979). The plate organs were counted “blind”, without knowing if the colonies were hygienic or non-hygienic (Table 2).
Statistical analysis The data were submitted to an analysis of variance for one dependent variable (number of sensilla placodea) by two factors (H = hygienic x NH = non-hygienic groups of bees and flagellomeres) and another variance analysis was made without grouping the colonies as hygienic or non-hygienic. Using the general linear model procedure, we tested the null hypotheses of the effect of the variables on the means of the groups of the single dependent variable (number of sensilla placodea). The interactions between the factors as well as the effects on individual factors were examined. The means of the number of sensilla placodea of flagellomeres 4, 6 and 8 were also compared with the Duncan test. RESULTS The mean number of plate organs was similar in the hygienic and non-hygienic colonies (P > 0.5, Table 2), though the variance was greater in the hygienic colonies. Colony number 5, with the highest mean, also had the highest rate of hygienic behavior (Table 2). There were significant differences in the number of sensilla placodea among the three flagellomeres (ANOVA, P < 0.05). Flagellomere number 4 had more plate organs than flagellomeres 6 and 8 (Duncan test, P < 0.05). DISCUSSION AND CONCLUSIONS In Apis mellifera (Stort and Rebustini, 1998), and in stingless bees (Meliponinae) (Stort and Moraes-Alves, 1998), there are greater numbers of sensory sensilla in the terminal segments or distal flagellomeres of the antennae (mainly number 10), decreasing in number in the proximal flagellomeres. In bees more primitive than Apis mellifera, such as the genus Ceratina, the differences are much more pronounced, as they have these sensilla structures only in the five distal flagellomeres (Stort, A.C., unpublished data). However, in our sample, flagellomere 4 had significantly more sensilla placodea than flagellomeres 6 and 8. We did not find significant differences in the number of sensilla placodea between the hygienic and non-hygienic bees (P > 0.5). However, the colonies of the hygienic group had a higher variability. Masterman et al. (2000) examined whether bees can differentiate natural from “abnormal” brood odors, as a mechanism involved in hygienic behavior. Using the proboscis extension reflex conditioning technique, they found that bees from hygienic and non-hygienic colonies are equally capable of differentiating flower odors. However, hygienic honey bees were significantly more efficient in distinguishing healthy from sick pupae infected with the fungus Ascosphaera apis. Although the most hygienic colony had the largest number of sensilla placodea and the three colonies with the largest number of these sensory organs were all in the hygienic colony group, the mean number of sensilla placodea was similar in the hygienic and non-hygienic colonies (Table 1). If these hygienic bees are more efficient at recognizing the odor of sick or damaged brood, then factors other than the number of sensilla placodea are probably involved. Possibly, hygienic bees are differentially more sensitive to odors associated with dead brood. Since hygienic and non-hygienic bees have been found to be equally sensitive to flower odors (Masterman et al., 2000), and we also found no significant differences in the number of antennal sensory organs, our original (alternative) hypothesis that hygienic bees have more sensilla placodea is probably incorrect. ACKNOWLEDGMENTS Our special thanks to Mr. João José dos Santos, technician of the Department of Genetics, FMRP-USP, for his beekeeping services. We also thank Marla Spivak for her suggestions and revision of this paper and the Institute of Chemistry, UNESP, Araraquara, for allowing us to use the scanning electron microscope. Research supported by CAPES. REFERENCES Anderson, D.L. and Trueman, J.W.H. (2000). Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp. Appl. Acarol. 24: 165-189. Aumeier, P. (1995). Untersuchungen zur olfaktorischen Wirtsfindung der parasitischen Bienenmilbe Varroa jacobsoni Oud. Diplomarbeit, Institut für Zoologie I, Abteilung Parasitologie der Friedrich, Alexander Universität Erlanger, Nürnberg, Germany. Aumeier, P., Rosenkranz, P. and Gonçalves, L.S. (1996). Abwehrmechanismen des Bienenvolkes gegenüber Varroatose und Brutkrankheiten: Ein Vergleich zwischen Apis mellifera carnica und Afrikanisierten Bienen in Brasilien. Apidologie 27: 286-288. Boecking, O. and Drescher, W. (1992). The removal response of Apis mellifera L. colonies towards sealed brood cells infested with Varroa jacobsoni: techniques, extent and efficacy. Apidologie 23: 371-373. Boecking, O. and Drescher, W. (1994). Rating of signals which trigger Apis mellifera L. bees to remove mite-infested brood. Apidologie 25: 459-461. Corrêa-Marques, M.H. and De Jong, D. (1998). Uncapping of worker bee brood, a component of the hygienic behavior of Africanized honey bees against the mite Varroa jacobsoni Oudemans. Apidologie 29: 283-289. De Jong, D. and Gonçalves, L.S. (1998). Las abejas africanizadas de Brasil se han vuelto tolerantes a Varroa. Apiacta 33: 65-70. De Jong, D., Gonçalves, L.S. and Morse, R.A. (1984). Dependence on climate of the virulence of Varroa jacobsoni. Bee World 65: 117-121. Dietz, A. and Humphreys, W.J. (1971). Scanning electron microscopic studies of antennal receptors of the worker honey bee, including sensilla campaniformia. Ann. Entomol. Soc. Am. 64: 919-925. Frisch, K. von (1921). Über den Sitz des Geruchsinnes bei Insekten. Zool. Jahrb. Abt. Allg. Zool. 38: 449-516. Gramacho, K.P. (1999). Fatores que interferem no comportamento higiênico das abelhas Apis mellifera. Ph.D. thesis, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto da Universidade de São Paulo, USP, Ribeirão Preto, SP, Brazil. Gramacho, K.P. and Gonçalves, L.S. (1994). Estudo comparativo dos métodos de congelamento e perfuração de crias para avaliação do comportamento higiênico em abelhas africanizadas. Anais do IV Congreso Iberolatinoamericano de Apicultura, Rio Cuarto-Córdoba, Argentina, pp. 45. Gramacho, K.P., Gonçalves, L.S. and Rosenkranz, P. (1998). Study of the temperature of brood killed by the pin-killing method in worker bees of Apis mellifera carnica. Apiacta 33: 33-41. Guerra Jr., J.C.V., Gonçalves, L.S. and De Jong, D. (2000). Africanized honey bees (Apis mellifera L.) are more efficient at removing worker brood artificially infested with the parasitic mite Varroa jacobsoni Oudemans than are Italian bees or Italian/Africanized hybrids. Genet. Mol. Res. 23: 89-92. Kaissling, K.E. and Renner, M. (1968). Specialized chemoreceptors in the pore plates of Apis. Z. Vergl. Physiol. 59: 357-361. Kulincevic, J.M., Rinderer, T.E. and Urosevi, D.J. (1988). Seasonality and colony variation of reproduction and non-reproduction Varroa jacobsoni females in western honey bee (Apis mellifera) worker brood. Apidologie 20: 173-180. Kuwabara, M. and Takeda, K. (1956). On the hygroreceptor of the honey bee Apis mellifera. Phys. Ecol. 7: 1-6. Masterman, R., Smith, B.H. and Spivak, M. (2000). Brood odor discrimination abilities in hygienic honey bees (Apis mellifera L.) using proboscis extension reflex conditioning. J. Insect Behav. 13: 87-101. McIndoo, N.E. (1914). The olfactory sense of the honey bee. J. Exp. Zool. 16: 265-346. Moretto, G., Gonçalves, L.S., De Jong, D. and Bichuette, M.Z. (1991). The effects of climate and bee race on Varroa jacobsoni Oud. Infestations in Brazil. Apidologie 22: 197-203. Moritz, R.F.A. (1998). A reevaluation of the two-locus model for hygienic behavior in honeybees (Apis mellifera L.). J. Hered. 79: 257-262. Newton, D.C. and Ostasiewski Jr., N.J. (1986). A simplified bioassay for behavioral resistance to American foulbrood in honey bees (Apis mellifera L.). Am. Bee J. 126: 278-281. Peng, Y.S., Fang, Y., Xu, S., Ge, L. and Nasr, M.E. (1987). Response of foster Asian honey bee (Apis cerana Fabr.) colonies to the brood of European honey bee (Apis mellifera L.) infested with parasitic mite Varroa jacobsoni Oudemanns. J. Invertebr. Pathol. 49: 259-264. Rosenkranz, P., Tewarson, N.C., Singh, A. and Engels, W. (1993). Differential hygienic behavior towards Varroa jacobsoni in capped worker brood of Apis cerana depends on alien scent adhering to the mites. J. Apic. Res. 32: 89-93. Rothenbuhler, W.C. (1964a). Behavior genetics of nest cleaning in honey bees. I. Responses of four inbreed lines to disease-killed brood. Anim. Behav. 12: 578-583. Rothenbuhler, W.C. (1964b). Behavior genetics of nest cleaning in honey bees. IV. Responses of F1 and backcross generations to disease-killed brood. Am. Zool. 4: 111-123. Sasagawa, H., Matsuyama, S. and Peng, C.Y.S. (1999). Recognition of parasite, hygienic allo-grooming behavior induced by parasitic Varroa mites in the Japanese honey bee, Apis mellifera japonica Rad. Proceedings of the XIII International Congress of IUSSI, Adelaide, Australia, pp. 415. Schultz-Langner, E. (1960). Zum verhalten der honigbiene beim säubern von zellen mit faulbrutkromken larven. Z. Bienenforsch. 5: 1-7. Snodgrass, R.E. (1935). Principles of Insect Morphology. McGraw-Hill Book Company, London, UK. Snodgrass, R.E. (1956). Anatomy of the Honey Bee. Comstock Publishing Associates, Ithaca, NY, USA. Spivak, M. (1996). Honey bee hygienic behavior and defense against Varroa jacobsoni. Apidologie 27: 245-260. Spivak, M. and Downey, D. (1998). Field assays for hygienic behavior in honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 91: 64-70. Stort, A.C. (1979). Estudo genético de caracteres morfológicos e suas relações com o comportamento de defesa de abelhas do gênero Apis. Associate Professor thesis, Instituto de Biociências de Rio Claro, UNESP, Rio Claro, SP, Brazil. Stort, A.C. and Moraes-Alves, M.M.B. (1998). A study of the sensory structures of the antennae of Scaptotrigona postica workers (Hymenoptera-Apidae). Rev. Bras. Biol. 58: 163-167. Stort, A.C. and Rebustini, M.M.E. (1998). Differences in the number of some antennal sensilla of four honey bee (Apis mellifera) types and comparisons with the defensive behaviour. J. Apic. Res. 37: 3-10. Titera, D. and Kokoris, J. (1994). Effect of the microinjections into the brood cells on the opening and cleaning behaviour of honeybees. Apidologie 25: 503-504. Warnke, U. (1976). Effects of electric changes on honeybees. Bee World 57: 50-56. |
|