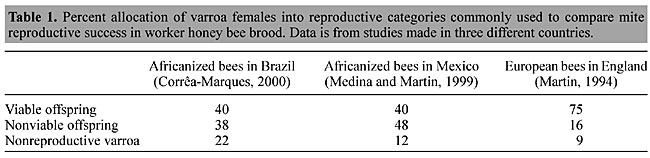
ABSTRACT. Varroa destructor reproductive success is considered an important character for determining the resistance of honey bees to this mite parasite. However, most of the published data are not comparable due to the different methods of ascertaining and reporting reproduction. A recently published technique that involves reconstructing mite families in older worker brood gives repeatable and reliable parameters. This methodology was used to compare various categories of reproduction of approximately 1,000 V. destructor females in each of three studies on Africanized bees in Brazil and Mexico and European bees in England. The most objective and useful measure was the determination of the number of viable females per female that had invaded the worker brood in singly infested cells, which was denominated the “effective reproduction rate”. Viable females are those that can reach the adult stage and have a mate available. The effective reproduction rate in worker brood was 0.64, 0.73 and 1.01 in Brazil, Mexico and England, respectively. Standardization of reproduction determination techniques would make published data comparable and much more useful. Key words: Varroa, Apis mellifera, Reproduction, Fertility, Africanized, Effective reproduction INTRODUCTION Although the mite Varroa destructor (formerly named Varroa jacobsoni; Anderson and Trueman, 2000) is widely recognized as the most important problem for apiculture throughout the world (De Jong, 1997), little is known about why it is lethal for colonies throughout most of the world, requiring treatment with chemicals, while in other regions, such as Brazil and some other parts of tropical America, the bees are maintained without the need for treatment (Rosenkranz, 1999). This tolerance to mite infestation appears to be influenced by climate (De Jong et al., 1984; Moretto et al., 1991) and bee race (Camazine, 1986; Rosenkranz, 1986; Moretto et al., 1993) as honey bees in tropical America are normally little affected, and Africanized bees are more tolerant than European races of bees. Another factor that may have an important influence on varroa mite virulence is the genetic variety of varroa present in the honey bee colonies. Anderson (2000) has indicated that there are two mitochondrial haplotypes of Varroa destructor. The Japanese/Thai haplotype apparently is not seriously damaging, while the Korean/Russian haplotype kills the Apis mellifera colonies that it attacks. However, Anderson and Trueman (2000) reported finding both haplotypes in Brazil, which is unexpected as there are no reports of colony mortality due to varroa in this country. A key factor, which appears to be correlated with the infestation levels in the colonies, is the fertility of the mites (De Jong, 1984). The reproductive capacity of the Varroa mite varies among the various honey bee races (Martin et al., 1997); however, objective comparisons among published studies are nearly impossible as each researcher has used different, often unspecified techniques (De Jong, 1997). Frequently the methodology is not clearly explained. We consider “viable offspring” the progeny that includes one live adult male mite and at least one live adult female mite (Medina and Martin, 1999). This is critical, since for a female mite to produce any offspring (even males) it must be mated (Harris and Harbo, 1999). So, although mature unmated females will become part of the overall mite population, they are unable to contribute to its subsequent growth. An estimate can be made of the mean number of progeny produced by the mother mites. This is called the “total reproductive rate”, which is equal to the number of eggs produced. This information can be used to help predict the number of offspring that will attain adulthood when the bee emerges. Examining older honey bee worker brood (more than 230 h after eclosion from the egg) is more accurate since it takes into account mite offspring mortality, which greatly affects the number of viable female offspring produced (Medina and Martin, 1999). In the regions of the world where varroa is not a problem, the percentage of adult female mites that can be considered fertile is reduced, when compared to places like Europe and North America, where reproductive capacity is high and untreated colonies die. Reduced reproduction has been reported from Uruguay (Ruttner et al., 1984) and Brazil (Ritter and De Jong, 1984). Only about 50% of the varroa mites found in worker brood cells in Brazil reproduce, whereas in northern Europe, more than 80% do so (Ritter and De Jong, 1984; Rosenkranz and Engels, 1994). Overall reproductive capacity may be affected by other factors, such as preferential hygienic uncapping of brood cells with reproducing mites (Corrêa-Marques and De Jong, 1998). It would be useful to know how important this and other characteristics are for determining the growth of varroa populations. Unfortunately, it is virtually impossible to objectively compare all of the various studies made on varroa reproduction, due to a lack of uniformity in the items analyzed and often because the methods are not fully explained. Consequently, it is not clear how differences in reproduction determine whether and to what degree this mite damages honey bee colonies in the different regions of the world. We compared recent data on varroa reproduction in Brazil with studies made in Mexico and England, and we examined the various reproductive parameters to determine which was best correlated with the impact of this mite on the honey bee colonies in each country. MATERIAL AND METHODS Varroa reproduction was studied in Africanized bee colonies in Ribeirão Preto, SP, Brazil, by a technique that involves the reconstruction of the original family groups (Martin, 1995). All of the infested brood cell contents are removed and observed with a stereomicroscope. Reconstruction is based on the supposition that a normal reproduction includes a single male as the first progeny, and subsequent females at 30-h intervals (Donze and Guerin, 1994; Martin, 1994). Analysis of mite debris for moulted skins and fecal patterns found within the cell aided in the determination of the reconstructed history of the mite family. This can be used to place the mites into reproductive categories and allows the reproductive rate of the mite to be calculated. However, it is necessary to standardize the calculation of these reproductive rates so that comparisons can be made between published studies. In order to do this, only those brood cells that are infested by a single female mite were used, since mites are less productive as the number of mother mites per cell increases (Fuchs and Langenbach, 1989). The female mites can then be grouped into different categories depending on their reproductive behavior and what is being investigated. The data from Brazil (Corrêa-Marques, 2000) were compared with information from two previous studies made in Mexico (Medina and Martin, 1999) and England (Martin, 1994). RESULTS AND DISCUSSION We initially compared the following mite reproduction categories: 1) mites producing viable offspring, 2) mites producing nonviable offspring, which includes mites producing only male offspring, and 3) mites producing no offspring or nonreproductive mites (Table 1). Viable offspring refers to cells that contain one live adult male mite and at least one adult female mite (Medina and Martin, 1999). This is important since for a mite to produce offspring, it must be mated (Martin et al., 1997; Harris and Harbo, 1999). Although mature unmated females will become part of the overall mite population, they are unable to contribute to its subsequent growth. There are various methods to calculate the reproductive rate of the varroa population and it must be clear how these have been derived if the reproductive values are to be compared across studies. An estimate can be made of the average number of progeny produced by the mother mites, which is often called the “total reproductive rate”. This information can be used to estimate the number of offspring that will attain adulthood when the bee emerges. Therefore, the total reproductive rate depends on the age distribution of the samples studied, and since the loss of offspring is normally not evaluated, the total reproductive rate is similar to the number of eggs produced. As mortality is not considered, this is the least reliable value with which to compare results from various studies.
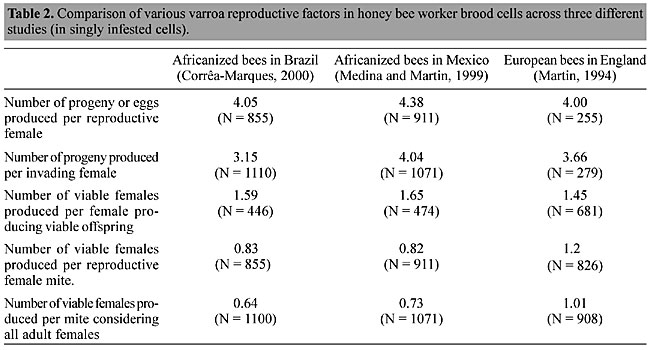
The percentage of mites with viable offspring was similar in Africanized bees in Mexico and Brazil, and was considerably higher in European bees in England (Table 1). Effectively, nonreproduction was mainly a consequence of nonviable offspring in all three countries. The fraction of completely nonreproductive female mites (those that produced no offspring) was above 20% only in Africanized bee colonies in Brazil. We also used three other measures: the average number of viable females produced by 1) the mothers that only produced viable female progeny, 2) all mothers that produced any progeny, and 3) all females that invaded bee brood cells. We suggest that this third measure is the most useful as it takes into account the nonreproducing mites, which are important for a determination of the real growth rate of the varroa population. We compared the results from these three studies using the above methods and found that the reproductive ability of mites in Brazil and Mexico was similar, when all the original females were included, while both were lower than that found in the UK (Table 2). We can now question the supposition that a less virulent Japan/Thailand haplotype of Varroa destructor is the main reason for the mite tolerance of Africanized bees (Anderson and Trueman, 2000), since in both the Mexican and UK studies the apparently more virulent Korean/Russian haplotype was present. Furthermore, according to a recent analysis of mites from the Brazilian apiary, they are also of the Korean/Russian haplotype (María Claudia Garrido Lüneburg, personal communication).
When we compare the number of progeny (including eggs) produced per reproductive mite (those that produced any progeny), the Africanized bees in Mexico had the highest rate, and the European bees in England the lowest, although quite similar to that found in Brazil (Table 2), which is unexpected when we consider that varroa is lethal for colonies in England, and not so in Brazil and Mexico (De Jong, 1997). Similar discrepancies were found when the number of progeny per invading mite and the number of “viable females” produced per female producing viable offspring were compared (Table 2). The only classifications that made sense, given the varroa situation in each country, were the number of viable females produced per reproductive female mite, and the number of viable females produced per invading female mite, considering all singly infested cells. Standardization of reproduction determination techniques has allowed us to make more objective comparisons and should lead to a better understanding of differences in varroa reproduction associated with various kinds of bees, climates and management techniques. REFERENCES Anderson, D.L. (2000). Variation in the parasitic bee mite Varroa jacobsoni Oud. Apidologie 31: 281-292. Anderson, D.L. and Trueman, J.W.H. (2000). Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp. Appl. Acarol. 24: 165-189. Camazine, S. (1986). Differential reproduction of the mite Varroa jacobsoni (Mesostigmata: Varroidae) on Africanized and European honey bees (Hymenoptera: Apidae). Ann. Entomol. Soc. Am. 79: 801-803. Corrêa-Marques, M.H. (2000). Sucesso reprodutivo do ácaro Varroa jacobsoni em abelhas Africanizadas (Apis mellifera). IV Encontro de Abelhas, Ribeirão Preto, SP, Brazil, pp. 228-232. Corrêa-Marques, M.H. and De Jong, D. (1998). Uncapping of worker bee brood, a component of the hygienic behavior of Africanized honey bees against the mite Varroa jacobsoni Oudemans. Apidologie 29: 283-289. De Jong, D. (1984). Current knowledge and open questions concerning reproduction in the honey bee mite, Varroa jacobsoni. Adv. Invertebr. Reprod. 3: 547-553. De Jong, D. (1997). Mites: Varroa and other parasites of brood. In: Honey Bee Pests, Predators and Diseases (Morse, R.A. and Flottum, K., eds.). A.I. Root Co., Medina, OH, USA, pp. 279-327. Donze, G. and Guerin, P.M. (1994). Behavioural attributes and parental care of Varroa mites parasitizing honeybee brood. Behav. Ecol. Sociobiol. 34: 305-319. Fuchs, S. and Langenbach, K. (1989). Multiple infestation of Apis mellifera L. worker cells and reproduction in Varroa jacobsoni Oud. Apidologie 20: 257-266. Harris, J.W. and Harbo, J.R. (1999). Low sperm counts and reduced fecundity of mites in colonies of honey bees (Hymenoptera: Apidae) resistant to Varroa jacobsoni (Mesostigmata: Varroidae). J. Econ. Entomol. 92: 83-90. Martin, S.J. (1994). Ontogenesis of the mite Varroa jacobsoni Oud. in worker brood of the honeybee Apis mellifera L. under natural conditions. Exp. Appl. Acarol. 18: 87-100. Martin, S.J. (1995). Reproduction of Varroa jacobsoni in cells of Apis mellifera containing one or more mother mites and the distribution of these cells. J. Apic. Res. 34: 187-196. Martin, S.J., Holland, K. and Murray, M. (1997). Non-reproduction in the honeybee mite Varroa jacobsoni. Exp. Appl. Acarol. 21: 539-549. Medina, L.M. and Martin, S.J. (1999). A comparative study of Varroa jacobsoni reproduction in worker cells of honey bees (Apis mellifera) in England and Africanized bees in Yucatan, Mexico. Exp. Appl. Acarol. 23: 659-667. Moretto, G., Gonçalves, L.S. and De Jong, D. (1991). The effects of climate and bee race on Varroa jacobsoni Oud. infestations in Brazil. Apidologie 22: 197-203. Moretto, G., Gonçalves, L.S. and De Jong, D. (1993). Heritability of Africanized and European honey bee defensive behavior against the mite Varroa jacobsoni. Rev. Bras. Genet. 16: 71-77. Ritter, W. and De Jong, D. (1984). Reproduction of Varroa jacobsoni in Europe, the Middle East and tropical South America. Z. Angew. Entomol. 98: 55-57. Rosenkranz, P. (1986). Factors affecting Varroa reproduction in colonies of Apis mellifera: a comparison of European and Africanized honey bees. Varroa Workshop, Feldafing, Germany, p. 16. Rosenkranz, P. (1999). Honey bee (Apis mellifera L.) tolerance to Varroa jacobsoni Oud. in South America. Apidologie 30: 159-172. Rosenkranz, P. and Engels, W. (1994). Infertility of Varroa jacobsoni females after invasion into Apis mellifera worker brood as a tolerance factor against Varroatosis. Apidologie 25: 402-411. Ruttner, F., Marx, H. and Marx, G. (1984). Beobachtungen uber eine mogliche Anpassung von Varroa jacobsoni an Apis mellifera L. in Uruguay. Apidologie 15: 43-62. |
|