
ABSTRACT. The UAS/GAL4 ectopic expression system is widely used in Drosophila melanogaster for the overexpression of transgenes. This system operates under the assumption that the yeast transcription factor, GAL4, is inactive in D. melanogaster. Thus, GAL4 can be expressed under the control of D. melanogaster-specific promoters with little effect upon the organism. We have shown that expression of GAL4 in the developing eye under the control of the glass multiple reporter (GMR) promoter element does have an effect on eye development. Although GMR-GAL4 heterozygotes appear normal when raised at 25°C, the homozygotes have a highly disorganized ommatidial array. In addition, the levels of apoptosis in the third-instar larval eye imaginal disc (where GAL4 is expressed) are slightly higher in GMR-GAL4 heterozygotes, and much higher in GMR-GAL4 homozygotes when compared to wild type discs. The morphological eye defects caused by GMR-GAL4 are significantly enhanced when flies are raised at 29°C (presumably due to the higher activity of GAL4 at this temperature); however, the levels of apoptosis appear to be similar at these two temperatures. Taken together, these data suggest that GAL4 can have adverse effects on D. melanogaster development, especially at high expression levels. In addition, GAL4 appears to induce apoptosis even in the absence of any visible morphological defects. Thus, despite the benefits of the UAS/GAL4 ectopic expression system, one must use caution in the design and interpretation of experiments. Key words: Drosophila melanogaster, Eye development, Apoptosis, UAS/GAL4 ectopic expression system INTRODUCTION The UAS/GAL4 ectopic expression system (Brand and Perrimon, 1993; reviewed in Brand et al., 1994; Phelps and Brand, 1998) has become a widely used and extremely valuable tool for the overexpression of transgenes in Drosophila melanogaster. This bipartite expression system utilizes the yeast transcription factor, GAL4, and its target sequence, UAS (upstream activation sequence), to which GAL4 binds in order to activate gene transcription. GAL4 can be expressed in many different patterns by placing it under the control of various D. melanogaster promoter sequences. Since UAS promoter sequences (CGGAGTACTGTCCTCC) are not found in D. melanogaster (Berkeley Drosophila Genome Project, personal communication), it is assumed that GAL4 is inactive. This is very useful for the overexpression of transgenes from a UAS promoter because GAL4 will drive expression of the transgene while not otherwise affecting the cells. Many overexpression studies in D. melanogaster have been carried out with the developing eye using the glass multiple reporter (GMR)-GAL4 driver line (Freeman, 1996). Flybase currently lists 111 articles that have used the construct since its creation in 1996. The GMR element causes high-level expression in the eye imaginal discs in cells posterior to the morphogenetic furrow. We have shown that GMR-GAL4 causes developmental defects and increased apoptosis in the eye, thus contradicting the notion that GAL4 is inactive in D. melanogaster. MATERIAL AND METHODS Fly stocks and culture GMR-GAL4 flies (Freeman, 1996) were obtained from the Bloomington Drosophila Stock Center at Indiana University and w1118 was obtained from Dr. Howard Lipshitz. GMR-GAL4 homozygous females were crossed to w1118 males and cultured on standard (cornmeal/yeast/molasses/agar) media at 25° and 29°C. The cultures of GMR-GAL4 and w1118 were also cultured at 25° and 29°C and all cultures were transferred to a new vial every two days to avoid overcrowding. Analysis of adult eyes and imaginal discs For scanning electron microscopy (SEM), two-day-old females were desiccated overnight and coated in gold before photography with a Hitachi S-570 SEM. Acridine orange was used to visualize apoptotic cells in the eye imaginal discs, as described by Bonini (2000). Third-instar larvae were dissected in phosphate-buffered saline (PBS) at pH 7.4 and imaginal discs were incubated for 5 min in 5 mg/ml acridine orange solution. Discs were then rinsed in PBS and photographed immediately using a Nikon Eclipse E600 fluorescent microscope. RESULTS AND DISCUSSION The development of the Drosophila eye is a complex, yet relatively well-understood process (reviewed in Baker, 2001). As a result, the eye is an excellent model for the study of genes involved in the control of developmental processes, such as cell proliferation, differentiation and apoptosis (Thomas and Wassarman, 1999). The UAS/GAL4 ectopic expression system has made it possible to express genes specifically in the developing eye and test the effects of overexpression on eye development. The GMR-GAL4 driver is a commonly used construct for the misexpression of transgenes in the developing eye. It has been reported that GMR-GAL4 homozygotes have a rough eye phenotype that is visible under the dissecting microscope (Freeman, 1997; Helms et al., 1999; Hiesinger et al., 1999; White and Jarman, 2000). Upon SEM analysis of GMR-GAL4 homozygotes raised at 25°C, it was revealed that the ommatidial array was disrupted due to the presence of irregularly sized ommatidia (Figure 1C). This phenotype is reminiscent of that obtained through expression of the small G-protein, Dras2 (Brand and Perrimon, 1993). GMR-GAL4 heterozygotes raised at 25°C do not have an obvious phenotype (Figure 1B) when compared to a control (Figure 1A), indicating that the effects of GAL4 may be dependent upon the gene dosage. When flies are raised at 29°C the phenotypic effects of GMR-GAL4 are enhanced (Figure 1G-I). At this temperature GMR-GAL4 homozygotes have grossly deformed eyes (Figure 1I) and the heterozygotes show a slight abnormality (Figure 1H), whereas the control eyes still appear normal (Figure 1G). The increased phenotypic severity seen at 29°C may be due to the higher activity of GAL4 at this temperature (Brand et al., 1994). Thus, we conclude that GMR-GAL4 causes developmental abnormalities in a dose- and temperature-sensitive manner. Acridine orange staining of the eye imaginal discs revealed high levels of apoptosis in GMR-GAL4 homozygotes (Figure 1F and L) and intermediate levels in the heterozygotes (Figure 1E and K), when compared to controls (Figure 1D and J). Apoptotic cells are brightly fluorescent. Note that the increased staining of apoptotic cells is only apparent posterior to the morphogenetic furrow where GAL4 is being expressed. Unlike the phenotype seen in the adult eye, there was no apparent difference in the levels of apoptosis between flies cultured at 25° and 29°C (Figure 1, compare D-F to J-L). Thus, induction of apoptosis by GAL4 appears to be dose sensitive, but not temperature sensitive, and can occur in the absence of a visible adult phenotype. The induction of apoptosis by GMR-GAL4 may be indirect, i.e., apoptosis occurs as a result of developmental confusion caused by high levels of GAL4 and not through direct regulation of genes involved in apoptosis. For example, the D. melanogaster nucleoporin, members only (mbo), is required for nuclear import of GAL4 (Uv et al., 2000), thus it is possible that high levels of GAL4 block the normal shuttling of molecules in and out of the nucleus. However, the temperature sensitivity of the GMR-GAL4 rough eye phenotype may indicate that GAL4 is causing direct transcriptional activation of certain genes. If the phenotypic enhancement at 29°C is due to the innate properties of GAL4 at this temperature, then GAL4 may be directly activating gene transcription in order to produce the phenotype. Alternatively, increased temperature may cause increased expression of GAL4, which could also enhance the phenotype. Regardless of the mechanism by which GAL4 disrupts eye development, it is apparent that there is an effect, and that apoptosis is increased. Thus, it is imperative that experiments be well controlled and that new GAL4 constructs be well characterized to avoid misinterpretation of results, especially for the study of apoptosis. 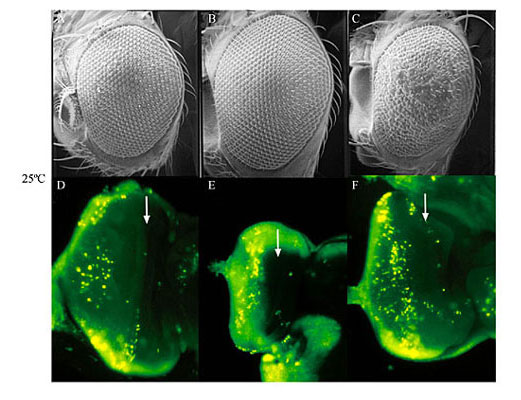 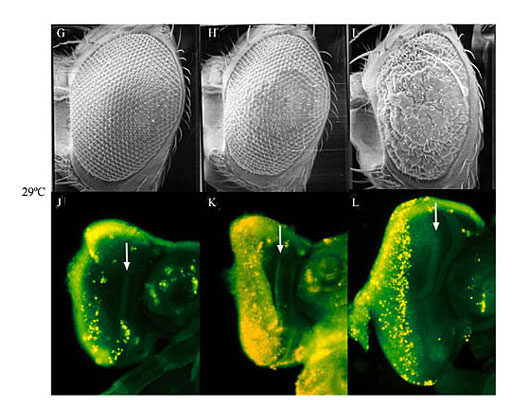 Figure 1. Expression of GAL4 under the control of the glass multiple reporter (GMR) promoter element causes developmental defects and apoptosis in the Drosophila melanogaster eye. Scanning electron microscopy reveals the phenotypic effect of GMR-GAL4 at 25°C (A-C), and 29°C (G, H). Acridine orange was used to stain apoptotic cells in the third-instar eye imaginal discs from larvae raised at 25°C (D-F) and 29°C (J-L). Genotypes are w1118 (A, D, G, H), w; GMR-GAL4/+ (B, E, H, K), and w; GMR-GAL4/GMR-GAL4 (C, F, I, L). Eyes are shown at 90X magnification and imaginal discs are shown at 125X magnification. Arrows indicate the location of the morphogenetic furrow in the imaginal discs. ACKNOWLEDGMENTS We would like to offer sincere thanks to Roy Ficken and Lisa Lee from the Department of Biology at Memorial University of Newfoundland (MUN) for their technical expertise. The work of B.E. Staveley was funded by the Natural Sciences and Engineering Research Council of Canada, the Banting Research Foundation and the Dean of Science of Memorial University of Newfoundland (start-up funds). J.M. Kramer was partially funded by the School of Graduate Studies at MUN. Thanks also to Dr. Howard Lipshitz at the Hospital for Sick Children in Toronto and to the Bloomington Drosophila Stock Center at Indiana University for providing fly stocks. REFERENCES Baker, N.E. (2001). Cell proliferation, survival, and death in the Drosophila eye. Semin. Cell Dev. Biol. 12: 499-507. Bonini, N.M. (2000). Methods to detect patterns of cell death in Drosophila. Methods Mol. Biol. 136: 115-121. Brand, A.H. and Perrimon, N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401-415. Brand, A.H., Manoukian, A.S. and Perrimon, N. (1994). Ectopic expression in Drosophila. Methods Cell Biol. 44: 635-654. Freeman, M. (1996). Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell 87: 651-660. Freeman, M. (1997). Personal communication to FlyBase available from http://flybase.bio.indiana.edu/.bin/fbpcq.html?FBrf0091569 Helms, W., Lee, H., Ammerman, M., Parks, A.L., Muskavitch, M.A. and Yedvobnick, B. (1999). Engineered truncations in the Drosophila mastermind protein disrupt Notch pathway function. Dev. Biol. 215: 358-374. Hiesinger, P.R., Reiter, C., Schau, H. and Fischbach, K.F. (1999). Neuropil pattern formation and regulation of cell adhesion molecules in Drosophila optic lobe development depend on synaptobrevin. J. Neurosci. 19: 7548-7556. Phelps, C.B. and Brand, A.H. (1998). Ectopic gene expression in Drosophila using the GAL4 system. Methods 14: 367-379. Thomas, B.J. and Wassarman, D.A. (1999). A fly’s eye view of biology. Trends Genet. 15: 184-190. Uv, A.E., Roth, P., Xylourgidis, N., Wickberg, A., Cantera, R. and Samakovlis, C. (2000). members only encodes a Drosophila nucleoporin required for rel protein import and immune response activation. Genes Dev. 14: 1945-1957. White, N.M. and Jarman, A.P. (2000). Drosophila atonal controls photoreceptor R8-specific properties and modulates both receptor tyrosine kinase and Hedgehog signalling. Development 127: 1681-1689. |
|