ABSTRACT. We have constructed a bacterial artificial chromosome (BAC) library for a European honey bee strain using the cloning enzyme HindIII in order to develop resources for structural genomics research. The library contains 36,864 clones (ninety-six 384-well plates). A random sampling of 247 clones indicated an average insert size of 113 kb (range = 27 to 213 kb) and 2% empty vectors. Based on an estimated genome size of 270 Mb, this library provides approximately 15 haploid genome equivalents, allowing >99% probability of recovering any specific sequence of interest. High-density colony filters were gridded robotically using a Genetix Q-BOT in a 4 x 4 double-spotted array on 22.5-cm2 filters. Screening of the library with four mapped honey bee genomic clones and two bee cDNA probes identified an average of 21 positive signals per probe, with a range of 7-38 positive signals per probe. An additional screening was performed with nine aphid gene fragments and one Drosophila gene fragment resulting in seven of the nine aphid probes and the Drosophila probe producing positive signals with a range of 1 to 122 positive signals per probe (average of 45). To evaluate the utility of the library for sequence tagged connector analysis, 1152 BAC clones were end sequenced in both forward and reverse directions, giving a total of 2061 successful reads of high quality. End sequences were queried against SWISS-PROT, insect genomic sequence GSS, insect EST, and insect transposable element databases. Results in spreadsheet format from these searches are publicly available at the Clemson University Genomics Institute (CUGI) website in a searchable format (http://www.genome.clemson.edu/projects/stc/bee/AM__Ba/). Key words: Bacterial artificial chromosome, BAC, Honey bee, Comparative genomics, BAC end sequencing, STC INTRODUCTION The honey bee (Apis mellifera L.) has long been important for honey production and for the pollination of crops. Yield increases of U.S. crops attributed to honey bee pollination were recently estimated at $14.6 billion (Morse and Calderone, 2000). Aside from its economic importance, this bee has been an important organism for behavioral studies. Interest in honey bees is a natural consequence of the long history of apiculture and of observations that have been made of activities of bees both outside and within the colony. Recently, the western honey bee has been of great interest primarily in two areas. Behavioral scientists have used this bee to study division of labor, communication, and the evolution of sociality. Neurobiologists and behaviorists have also used the honey bee as a model organism for studying the molecular basis of learning (Menzel, 1983, 1990, 1999; Menzel and Müller, 1996; Erber et al., 2000). As a social insect, the honey bee lives in colonies with a single reproductive female, the queen that normally mates with 7-20 males (drones) and stores the sperm in a spermatheca (Adams et al., 1977; Estoup et al., 1994). Bees and other insects of the order Hymenoptera are haplodiploid. Mated queens have the ability to lay unfertilized eggs. But if the cell of the honeycomb that a queen inspects is small in width, then the egg is fertilized as it passes down the oviduct. Fertilized eggs become nonreproductive females (workers). The queen lays unfertilized eggs in the larger “drone cells” and these eggs develop parthenogenetically as haploid drones. How natural selection acts on a genome that is alternatively expressed in haploid and diploid individuals is an interesting evolutionary question. A related question is the pattern of gene expression in male and female bees. Since the workers perform all of the tasks necessary for the maintenance and growth of the colony and only workers are diploid, many genes may be sex-limited in expression. In addition to haploid males, honey bees offer some other interesting advantages for genetic analysis. Artificial insemination is a routine technique with the honey bee (Laidlaw and Page, 1997). The bee has a genome that is only a little larger than that of Drosophila (about 270 Mb; Jordan and Brosemer, 1974; Crain et al., 1976). Bees have only 8-10% repetitive DNA (Jordan and Brosemer, 1974; Crain et al., 1976). It also has been reported that honey bees have a higher rate of meiotic recombination than any known for a metazoan (Hunt and Page, 1995; Beye et al., 1999). The high recombination rate effectively increases the accuracy of linkage mapping. The high recombination rate and the low incidence of repetitive DNA should facilitate map-based cloning of genes in the honey bee. The behavior of the honey bee has been more thoroughly studied than its genetics. There is a division of labor in bee colonies that arises from age-based behavioral progression through various tasks (Lindauer, 1953a,b). But division of labor is also influenced by genetic predisposition to perform certain tasks. Recently, there have been attempts to explain aspects of division of labor based on physiology, neurobiology and genetics (Page and Robinson, 1990; Calderone and Page, 1991; Huang and Robinson, 1999; Page and Erber, 2002). Detailed linkage maps of the honey bee genome have been constructed for use in behavioral genetics studies (Hunt and Page, 1995; Hunt et al., 1998; Page et al., 2000; Guzmán-Novoa et al., 2002). Male haploidy can be used as a tool to study the genetics of behavioral traits in bees because the drone transmits the same genome to all of his worker progeny, and haploids facilitate genetic analyses. By backcrossing individual drones from an F1 queen to one of the parental lines, whole-colony phenotypes can be compared in a segregating panel of colonies, and phenotypes can be correlated with the inheritance of specific markers from each drone father. Quantitative trait loci (QTL) that influence colony-level behavioral traits in bees have been mapped and most of these QTL have been confirmed in independent crosses (Hunt et al., 1995, 1998, 1999; Page et al., 1995, 2000). Recently, QTLs that influence learning performance have been mapped based on the performance of individual drones (Chandra et al., 2001). An essential tool for characterizing genomes is the availability of large-insert genomic libraries. The bacterial artificial chromosome (BAC) library system has become the primary tool for generating large insert libraries for both plants and animals. The BAC vectors from the mini-F plasmid allow cloning and stable maintenance of large DNA fragments in Escherichia coli (Shizuya et al., 1992). BAC libraries are popular for a number of reasons, including: ease of handling, relative simplicity to develop, and low frequency of chimeric clones (Shizuya et al., 1992). A number of BAC libraries have been developed for various domesticated animal species, such as bovine, dog, goat, horse, rabbit, sheep, and swine (Schibler et al., 1998; Goddard et al., 1998; Zhu et al., 1999; Li et al., 1999; Vaiman et al., 1999; Buitkamp et al., 2000; Anderson et al., 2000; Rogel-Gillaird et al., 1999, 2001; Fahrenkrug et al., 2001). Published reports of BAC libraries for important insect species are rare, although there are several for Drosophila and the silkworm (Wu et al., 1999; Hoskins et al., 2000). In this report, we describe the construction and characterization of a large insert genomic honey bee BAC library. MATERIAL AND METHODS BAC library construction The BAC vector pCUGI1 was utilized and prepared as described by Luo et al. (2001). Megabase bee DNA was obtained from haploid drone pupae from a single, inbred queen of European origin. Fresh bee pupae were placed in a 1X Dulbeccos phosphate-buffered saline solution (GibcoBRL, Grand Island, NY, USA) and gently macerated in a Dounce tissue grinder with a loose piston. Large debris were removed by passing the mixture through one layer of Miracloth. Cells were then adjusted to a concentration of approximately 5 x 108 cells per ml and imbedded in a 1:1 (v/v) ratio with 1.2% agarose (final concentration of 0.6%). The agarose plug mixture was solidified in 75 ml plug molds (Bio-Rad, Hercules, CA, USA) and processed with lysis buffer and proteinase K as described by Peterson et al. (2000). Partial digests of megabase DNA (using HindIII), size selections, and ligations were performed as described in detail by Peterson et al. (2000). Recombinant colonies were picked using the Genetix Q-bot and stored individually in one hundred ninety-two 384-well microtiter plates (Genetix). Three copies of the library were made and stored in separate -80°C freezers. BAC clone characterization To prepare BAC DNA, 3 ml LB chloramphenicol (12.5 mg/ml) cultures were grown overnight in 6-cell autogen tubes and miniprepped robotically (Autogen 740 plasmid isolation system). To estimate insert size and determine the distribution of clone size, 247 BAC preps were performed from clones selected at random throughout the library. The BAC DNA was digested with 7.5 units of NotI (10 h at 37°C) and analyzed by pulsed field electrophoresis in 1% agarose gels (6 v/cm, 5-15 s switch time, 15 h run time, 14°C). BAC library screening High-density colony filters for hybridization-based screening of the library were prepared using the Genetix Q-bot. Clones were gridded in duplicate using a 4 x 4 array on 22.5-cm square Hybond N+ filters (Amersham). This gridding pattern allows 18,432 clones to be represented per filter. Colony filters were treated and hybridized using standard techniques (Sambrook et al., 1989). Radiolabeling (32P) of probe DNA and hybridization of colony filters were performed using standard techniques (Sambrook et al., 1989). Four mapped honey bee genomic clones, two bee cDNAs, nine pea aphid growth/developmental gene fragments, and one Drosophila simulans gene fragment were used to screen the bee BAC filters. The probes and their descriptions are listed in Table 1. The bee probes were obtained from the laboratory of Greg Hunt (Purdue University) while the pea aphid and Drosophila probes were obtained from the laboratory of David Stern (Princeton University). Probes were prepared by radiolabeling (32P) gel purified plasmid inserts. Protocols for hybridizing high-density BAC colony filter arrays and determining addresses of positive signals are publicly available at the Clemson University Genomics Institute (CUGI) website (www.genome.clemson.edu).
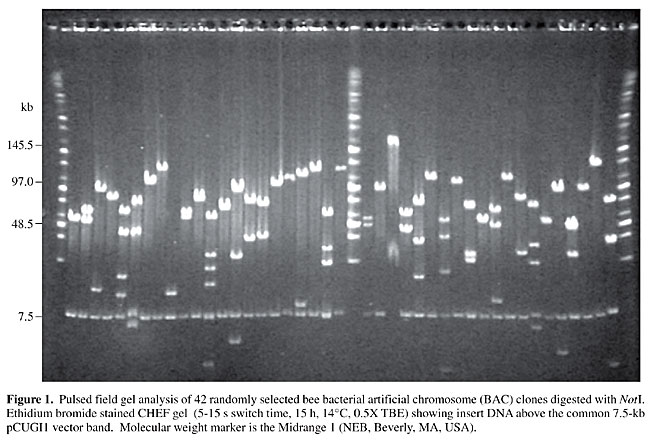
BAC end sequencing Preparation of BAC DNA for end sequencing was done in a 96-well format using standard alkaline lysis miniprep techniques. Sequencing reactions were set up according to manufacturer instruction for the Big Dye Terminator chemistry (Applied Biosystems). Reactions were performed using both forward and reverse universal primers. Samples were loaded onto 48-lane sequencing gels in ABI377 automated sequencers. Gels (250 ml) were composed of the following: 5% Long Ranger (FMC), 6 M urea, 18 ml TEMED, 150 ml ammonium persulfate (10% stock), and 1X TBE buffer. Reaction products were electrophoresed for 3.5 h. Base-calling was performed automatically using PHRED (Ewing and Green, 1998; Ewing et al., 1998), and vector sequences were removed by CROSS-MATCH (http://www.genome.washington.edu). High-quality BAC end sequences (defined as those having >100 nonvector bases with a PHRED quality value of 20 or greater) were used as queries in FASTX searches against SWISS-PROT, GenbankNR, insect genomic sequence GSS, insect expressed sequence tags (EST), Drosophila, and insect transposable element databases using a low-complexity sequence filter (in-house Perl script). All software was run locally on a Sun Ultra30 workstation using Solaris 2.6. Bee BAC end sequences have been submitted to GenBank under accession numbers BH814978 to BH817038. Database query results may be viewed and/or searched at the following CUGI web site: http://www.genome.clemson.edu/projects/stc/bee/AM__Ba/. RESULTS BAC library construction and characterization A BAC library was constructed for a European strain of honey bee that is suitable for physical mapping, sequence tagged connector (STC) development, and cloning genes associated with important agricultural and behavioral traits. The strain that was used is vouchered at the laboratory of R.E. Page (Department of Entomology, University of California, Davis, CA, USA). HindIII was used as the cloning enzyme. The library consists of 36,864 clones stored in ninety-six 384-well microtiter plates. Approximately 2% of the clones do not contain inserts, as judged by random analysis of BACs sampled from the library. A random sampling of 247 BACs indicated an average insert size of 113 kb with a range of 27 to 213 kb. Based on a haploid genome size of 270 Mb, the coverage of the library is approximately 15 haploid genome equivalents, resulting in a 99% probability of recovering any specific sequence. Figure 1 shows 42 randomly selected clones digested with NotI to release the insert. Because NotI is a GC-8-base cutter, digestion typically generates up to five insert bands, based on our data.
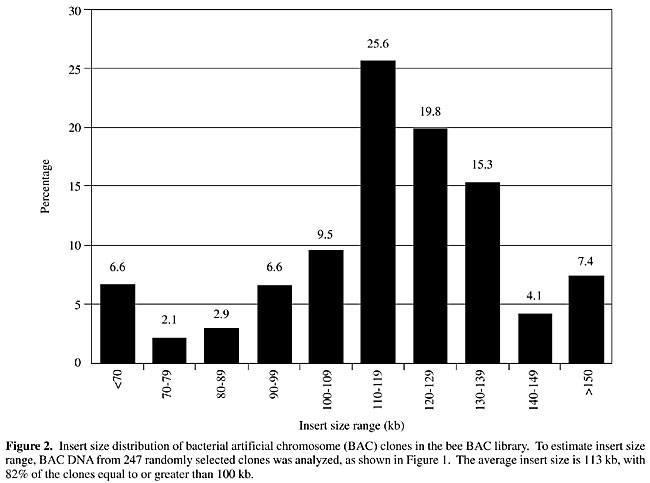
To determine the size distribution of BAC clones in the library, the 247 BACs analyzed by NotI digests were grouped by insert size and the insert size of each clone was plotted against the frequency of each group of clones represented in the library (Figure 2). A majority of the clones in the library (82%) are 100 kb or larger.
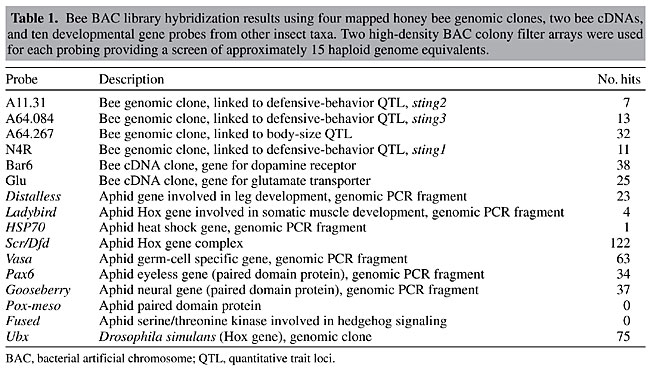
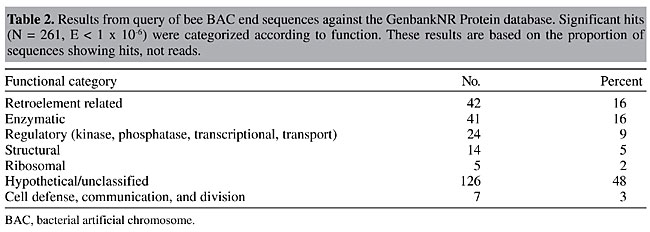
To test library coverage and isolate genomic regions putatively associated with important traits such as aggressive behavior and body size, two BAC colony filters (representing 15 haploid genome equivalents) were screened using purified inserts from six different bee probes (Table 1). Three of the bee probes were small-insert genomic clones linked to QTLs associated with aggressive behavior while another genomic clone was linked to a QTL for body size. Two more probes were bee cDNAs representing genes for a dopamine receptor and a glutamate transporter. These genes are likely to influence key neurological processes and behavior. The wide range of positive signals identified between probes (range = 7 to 38) may be indicative of the effects of preferential cloning obtained from the use of restriction enzymes. Nevertheless, an adequate number of positive clones was obtained for all of the bee probes. The average number of hits (21) was slightly above the expected estimate of 15 positive signals per probe, suggesting a higher frequency of HindIII sites in some of the targeted regions of the genome. The use of restriction endonucleases for DNA fragmentation in the construction of BAC libraries may involve preferential cloning of some genomic regions, and under-representation of other regions. A second set of hybridization experiments was performed to identify regions of the bee genome associated with growth and development. To do this, a set of nine pea aphid clones and one Drosophila clone were obtained from the laboratory of David Stern (Princeton University). All of the genomic clones were fragments of transcription factors and cell signaling genes associated with various aspects of insect development. Seven of the nine aphid probes and the Drosophila probe produced positive signals with a range of 1 to 122 positive signals per probe (average of 45). Some of the gene probes (e.g., Scr/Dfd and Ubx) obviously contained motifs common to various transcription factor gene families (eg., Hox sequences) and resulted in a large number of hits. Data from this hybridization experiment show that it is possible to identify homologous regions between highly divergent insect taxa. The identification of these BAC clones will allow for detailed studies in evolution and development by comparing large homologous genomic regions among diverse insect taxa. BAC end sequencing To examine the feasibility of using an STC strategy (Venter et al., 1996) to establish a framework for sequencing the bee genome, we sequenced and analyzed the ends (forward and reverse) of the first 1,536 clones in the library. A total of 3,072 sequencing reactions were performed giving 2,685 reads (success rate = 87%). High-quality sequences were defined as those having >100 high-quality bases other than the vector and E. coli sequences and a PHRED score of 20 or greater. The number of high-quality sequences was 2,061, with an average high-quality base count of 235. High-quality nonredundant sequences were searched against the GenbankNR database using the FASTX algorithm. A probability cutoff value (E value) of at least 10-6 was used to assign putative identities to the STCs. The GenbankNR search resulted in 261 (9.7%) of the sequences showing similarity to genes of known function. Significant search results were then sorted into seven different functional categories (Table 2). The largest grouping of hits was to hypothetical or unclassified proteins and represented nearly half (48%). The next largest group (16%) of the STCs shared sequences similar to insertion elements and encoded maturases, transposases, integrases, and various other viral type proteins. A roughly equivalent amount of STCs were those involved in metabolism (16%), followed by STCs involved in regulatory roles (9%), structural roles (5%), cell communication/division (3%), and ribosomal (2%). Of the highly significant STCs showing similarity to various proteins, 11% were best matches to Drosophila melanogaster proteins. The rest of the hits were to a wide variety of eukaryotic organisms. The bee STC query results of the GenbankNR database are publicly available at the CUGI website: (http://www.genome.clemson.edu/projects/stc/bee/AM__Ba/)
DISCUSSION We describe the development and characterization of a high-quality BAC library for the European honey bee. This large insert library provides an important resource for map-based cloning as well as for genome sequencing. The library has been deposited in the CUGI BAC/EST Resource Center and is publicly available. Requests for the library, high-density BAC colony filter arrays and clones can be made by accessing the CUGI web page (www.genome.clemson.edu). The utility of the library for identifying important regions of the genome associated with behavioral traits was demonstrated by screening the BAC colony filters with a variety of single-copy mapping probes and unique genes derived from honey bee DNA. The results were indicative of the good representation provided by the library, as an average of 21 hits per probing was obtained for each of the six different single-copy clones. Further, each probe identified no less than seven clones. Screening the library with genic probes derived from distant insect taxa was also highly effective as eight out of 10 probes were successful in identifying putative BACs associated with developmental genes. These results form the basis for using a hybridization-based approach to identify candidate BACs for genome sequencing among diverse insect taxa for detailed molecular studies in evolution and development. End sequencing of 1,536 BAC clones produced a nonredundant high-quality set of 2,061 STCs. Search results against the GenbankNR databases gave 261 significant hits (>1 x 10-6). Results were manually sorted according to function. Interestingly, the low number of database hits we observed in the search results was similar to the results reported previously in a recently published bee EST project (Whitfield et al., 2002). Given that the complete genomic sequence of Drosophila is now available, we were also surprised at the low levels of sequence similarity between the honey bee and this fruit fly and the large numbers of hits obtained on distantly related eukaryotes, primarily Chordata. Whitfield et al. (2002) also noted this trend with ESTs and concluded that this was due to a high level of divergence and gene loss events in Drosophila. The only conclusion that can be drawn at this point, based on both BAC end sequence and EST data, is that the bee genome is highly divergent. As more insect genomes are explored, the phylogenetics of Apis should become clearer. A recent announcement from the National Institutes of Health indicates that the genome of the European honey bee will be sequenced some time in the near future (http://www.genome.gov/page.cfm?pageID=10002851). In light of this announcement, our BAC library made from a European strain will provide a powerful tool to use in the study of bee behavior related to aggressiveness, honey production, foraging traits, and associative learning. ACKNOWLEDGMENTS Appreciation for technical assistance during the process of library replication and filter production is extended to Kerry Wilson and Stephanie Brown. Appreciation for the donation of bee pupae is extended to Martin Beye and M. Kim Fondrk (University of California, Davis). Research supported by funds from Purdue University (West Lafayette, IN), the Hayward Genetics Foudation and the National Science Foundation (NSF) Plant Genome grant # 9872676. REFERENCES Adams, J., Rothmann, E., Kerr, W.E. and Paulino, Z.L. (1977). Estimation of sex alleles and queen matings from diploid male frequencies in a population of Apis mellifera. Genetics 86: 583-596. Anderson, S.I., Lopez-Corrales, N.L., Gorick, B. and Archibald, A.L. (2000). A large-fragment porcine genomic library resource in a BAC vector. Mamm. Genome 11: 811-814. Beye, M., Hunt, G.J., Page, R.E., Fondrk, M.K., Grohmann, L. and Moritz, R.F.A. (1999). Unusually high recombination rate detected in the sex locus region of the honey bee (Apis mellifera). Genetics 153: 1701-1708. Buitkamp, J., Kollers, S., Durstewitz, G., Fries, R., Welzel, K., Schafer, K., Kellermann, A. and Lehrach, H. (2000). Construction and characterization of a gridded cattle BAC library. Anim. Genet. 6: 347-351. Calderone, N.W. and Page, R.E. (1991). Evolutionary genetics of division of labor in colonies of the honey bee (Apis mellifera). Am. Nat. 138: 69-92. Chandra, S.B., Hunt, G.J., Cobey, S. and Smith, B.H. (2001). Quantitative trait loci associated with reversal learning and latent inhibition in honeybees (Apis mellifera). Behav. Genet. 31: 275-285. Crain, W.R., Davidson, E.H. and Britten, R.J. (1976). Contrasting patterns of DNA sequence arrangement in Apis mellifera (honey bee) and Musca domestica (housefly). Chromosoma 59: 1-12. Erber, J., Pribbenow, B., Kisch, J. and Faensen, D. (2000). Operant conditioning of antennal muscle activity in the honey bee (Apis mellifera L.). J. Comp. Physiol. A 186: 557-565. Estoup, A., Solignac, M. and Cornuet, J.M. (1994). Precise assessment of the number of patrilines and genetic relatedness in honey bee colonies. Proc. R. Soc. Lond. B Biol. Sci. 258: 1-7. Ewing, B. and Green, P. (1998). Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 8: 186-194. Ewing, B., Hillier, L., Wendl, M. and Green, P. (1998). Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 8: 175-185. Fahrenkrug, S.C., Rohrer, G.A., Freking, B.A., Smith, T.P., Osoegawa, K., Shu, C.L., Catanese, J.J. and de Jong, P.J. (2001). A porcine BAC library with tenfold genome coverage: a resource for physical and genetic map integration. Mamm. Genome 12: 472-474. Goddard, S., Schibbler, L., Oustry, A., Cribiu, E.P. and Guerin, G. (1998). Construction of a horse BAC library and cytogenetical assignment of 20 type I and type II markers. Mamm. Genome 9: 633-637. Guzmán-Novoa, E., Hunt, G.J., Uribe, J.L., Smith, C. and Arechavaleta-Velasco, M.E. (2002). Confirmation of QTL effects and evidence of genetic dominance of honey bee defensive behavior: Results of colony and individual behavioral assays. Behav. Genet. 32: 95-102. Hoskins, R.A., Nelson, C.R., Berman, B.P., Laverty, T.R., George, R.A., Ciesiolka, L., Naeemuddin, M., Arenson, A.D., Durbin, J., David, R.G., Tabor, P.E., Bailey, M.R., DeShazo, D.R., Catanese, J., Mammoser, A., Osoegawa, K., de Jong, P.J., Celniker, S.E., Gibbs, R.A., Rubin, G.M. and Scherer, S.E. (2000). A BAC-based physical map of the major autosomes of Drosophila melanogaster. Science 288: 1751-1755. Huang, Z.-Y. and Robinson, G.E. (1999). Social control of division of labor in honey bee colonies. In: Information Processing in Social Insects (Denoubourg, J., ed.). Birhauser, Cambridge, MA, USA, pp. 165-186. Hunt, G.J. and Page, R.E. (1995). A linkage map of the honey bee, Apis mellifera, based on RAPD markers. Genetics 139: 1371-1382. Hunt, G.J., Page, R.E., Fondrk, M.K. and Dullum, C.J. (1995). Major quantitative trait loci affecting honey bee foraging behavior. Genetics 141: 1537-1545. Hunt, G.J., Guzmán-Novoa, E., Fondrk, M.K. and Page, R.E. (1998). Quantitative trait loci for honey bee stinging behavior and body size. Genetics 148: 1203-1213. Hunt, G.J., Collins, A.M., Riviera, R., Page, R.E. and Guzmán-Novoa, E. (1999). Quantitative trait loci for honey bee alarm pheromone production. J. Hered. 90: 585-589. Jordan, R.A. and Brosemer, R.W. (1974). Characterization of DNA from three bee species. J. Insect Physiol. 20: 2513-2520. Laidlaw, H.H. and Page, R.E. (1997). Queen Rearing and Bee Breeding. Wicwas Press, Cheshire, CT, USA. Li, R., Mignot, E., Faraco, J., Kadotani, H., Cantanese, J., Zhao, B., Lin, X., Hinton, L., Ostrander, E.A., Patterson, D.F. and de Jong, P.J. (1999). Construction and characterization of a eight-fold redundant dog genomic bacterial artificial chromosome library. Genomics 58: 9-17. Lindauer, M. (1953a). Division of labour in the honey bee colony. Bee World 34: 63-73. Lindauer, M. (1953b). Division of labour in the honey bee colony. Bee World 34: 85-90. Luo, M., Wang, Y., Frisch, D., Joobeur, T., Wing, R. and Dean, R. (2001). Melon BAC library construction using improved methods and identification of clones linked to the locus conferring resistance to melon Fusarium Wilt (Fom-2). Genome 44: 154-162. Menzel, R. (1983). Neurobiology of learning and memory: The honey bee as a model system. Naturwissenschaften 70: 504-511. Menzel, R. (1990). Learning, memory and congnition in honey bees. In: Neurobiology of Comparative Cognition (Kesner, R.P. and Olten, D.S., eds.). Lawrence Erlbaum, Hillside, NJ, USA, pp. 237-292. Menzel, R. (1999). Memory dynamics in the honey bee. J. Comp. Physiol. 185: 323-340. Menzel, R. and Müller, U. (1996). Learning and memory in honey bees: from behavior to neural substrates. Annu. Rev. Neurosci. 19: 379-404. Morse, R.A. and Calderone, N.W. (2000). The value of honey bees as pollinators of U.S. crops in 2000. Bee Cult. 128: 2-15. Page, R.E. and Erber, J. (2002). Levels of behavioral organization and evolution of division of labor. Naturwissenschaften 89: 91-106. Page, R.E. and Robinson, G.E. (1990). The genetics of division of labour in honey bee colonies. Adv. Insect Physiol. 23: 118-169. Page, R.E., Waddington, K.D., Hunt, G.J. and Fondrk, M.K. (1995). Genetic determinants of honey bee foraging behavior. Anim. Behav. 50: 1617-1625. Page, R.E., Fondrk, M.K., Hunt, G.J., Guzmán-Novoa, E., Humphries, M.A., Nguyen, K. and Greene, A. (2000). Genetic dissection of honey bee (Apis mellifera L.) foraging behavior. J. Hered. 91: 474-479. Peterson, D., Tomkins, J., Frisch, D., Wing, R. and Paterson, A. (2000). Construction of plant bacterial artificial chromosome (BAC) libraries: an illustrated guide. J. Agric. Genomics, Vol. 5. Available at http://www.ncgr.org. Rogel-Gillaird, C., Bourgeaux, N., Billault, A., Vaiman, M. and Chardon, P. (1999). Construction of a swine BAC library: application to the characterization and mapping of porcine type C endoviral elements. Cytogenet. Cell Genet. 85: 205-211. Rogel-Gillaird, C., Piumi, F., Billault, A., Bourgeaux, N., Save, C.C., Urien, C., Salmon, J. and Chardon, P. (2001). Construction of rabbit bacterial artificial chromosome library: application to mapping of the major histocompatibility complex to position 12q.1.1. Mamm. Genome 12: 253-255. Sambrook, J., Frisch, E. and Maniatus, T. (1989). Molecular Cloning: A Laboratory Manual. 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA. Schibler, L., Vaiman, D., Oustry, A., Guinec, N., Dangy-Caye, A.L., Billault, A. and Cribiu, E.P. (1998). Construction and extensive characterization of a goat bacterial artificial chromosome library with threefold genome coverage. Mamm. Genome 9: 119-124. Shizuya, H., Birren, B., Kim, U., Mancino, V., Slepak, T., Tachiiri, Y. and Simon, M. (1992). Cloning and stable maintenance of 300-kilobase-pair fragments of human DNA in Escherichia coli using an F-factor-based vector. Proc. Natl. Acad. Sci. USA 89: 8794-8797. Vaiman, D., Billault, A., Tabet-Aoul, K., Schibler, L., Vilette, D., Oustry-Vaiman, A., Soravito, C. and Cribiu, E.P. (1999). Construction and characterization of sheep BAC library of three genome equivalents. Mamm. Genome 10: 585-587. Venter, C., Smith, H.O. and Hood, L. (1996). A new strategy for genome sequencing. Science 381: 364-366. Whitfield, C.W., Band, M.R., Bonaldo, M.F., Kumar, C.G., Liu, L., Pardinas, J.R., Robertson, H.M., Soares, M.B. and Robinson, G.E. (2002). Annotated expressed sequence tags and cDNA microarrays for studies of brain and behavior in the honey bee. Genome Res. 12: 555-566. Wu, C., Asakawa, S., Shimizu, N., Kawasaki, S. and Yasukochi, Y. (1999). Construction and characterization of bacterial artificial chromosome libraries from the silkworm, Bombyx mori. Mol. Gen. Genet. 261: 698-706. Zhu, B., Smith, J.A., Tracey, S.M., Konfortov, B.A., Welzel, K., Schalkwyk, L.C., Lehrach, H., Kollers, S., Masabanda, J., Buitkamp, J., Fries, R., Williams, J.L. and Miller, J.R. (1999). A 5X genome coverage bovine BAC library: production, characterization, and distribution. Mamm. Genome 10: 706-709. |