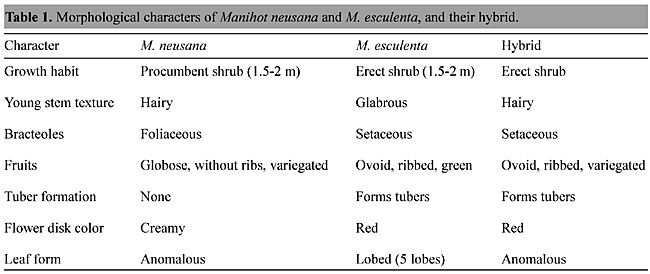
ABSTRACT. About 98 species of Manihot are known. All of them are native to the New World and are concentrated in four regions in Brazil and Central America. All the Manihot species so far examined have 2n = 36 chromosomes. Interspecific hybrids between cassava and its wild relatives show relatively normal meiosis, and further generations can be obtained. Electrophoresis shows affinity among wild species of different sections, and between some of them and cassava. Both polyploidy and apomixis may have contributed to speciation in this genus. Polyploidy produced genetic variability, while apomixis is responsible for perpetuating new hybrid types adapted to different environments. Cassava may have originated by hybridization between two wild Manihot species, followed by vegetative reproduction of the hybrid. Key words: Centers of diversity, Apomixis origin, Electrophoresis, Evolution, Interspecific hybridization, Polyploidy, Unreduced gametes INTRODUCTION Cassava, Manihot esculenta Crantz (Euphorbiaceae), is one of the leading food and feed plants of the world. It ranks fourth among staple crops, with a global production of about 160 million tons. Most of this is grown in three regions: West Africa and the adjoining Congo basin, tropical South America and south and southeast Asia. Cassava is drought resistant and grows well in poor soil. It is one of the most efficient producers of carbohydrates and energy among all the food crops (Vries et al., 1976). Vavilov (1951) assumed that the center of diversity of cassava is in the Brazilian-Bolivian region. He proposed that centers of diversity are the places of origin of cultivated plants. Since the idea of center of diversity was proposed in the 1920’s, much more information has been gathered, and it is now believed that not all centers of diversity represent centers of origin. Harlan (1961) showed that more than one center of diversity may be formed for a crop through introgression. This phenomenon can explain why in many cases centers of diversity for a given crop are found far from areas of high diversity of wild relatives. Since Harlan proposed this theory, with a convincing example of evolved species of Helianthus, much evidence has supported it. Dobzhansky (1973) indicated many conspicuous cases, such as the formation of species of Iris, Eucalyptus, Liatris, Penstemon and Tragopogon. This phenomenon serves as a model for what apparently happened in the formation of the four centers of diversity in Manihot (Nassar, 1978b). Assuming that cassava was domesticated for the first time in one place, it was then carried by Indians along with their migrations; extensive hybridization between the cultivated species and local wild ones might have taken place, giving rise to numerous new species. Cassava does not grow wild (Rogers and Appan, 1973). The large variation found in cassava (Rogers and Fleming, 1973) may be due to its having been maintained by vegetative reproduction during hundreds of years. Hybrids between some wild species may have been domesticated and maintained afterwards through vegetative reproduction. This assumption is supported by the fact that many experimental crosses involving cultivated M. esculenta led to hybrids between it and local wild species (Lanjouw, 1939; Nichols, 1947; Bolhuis, 1953; Jennings, 1959; Cruz, 1968; Abraham, 1975; Nassar, 1980a, 1989, 1991; Nassar et al., 1986). Genetic and cytological barriers between species do not appear to be well established in this genus systems. Schmidt’s (1951) observation about the very rapid response obtained through selection for increased starch content in tubers and tuber formation in just a few generations, in various wild species, supports this proposition. It would appear that many different wild species possess the potentiality to increase tuber formation and starch content. For instance, the two tree species of Manihot (M. epruinosa and M. brachyandra) frequently grown in courtyards in Goiânia produce many tubers. These two species are native to Bahia. They may have been introduced by the people of this state. It seems that they were carried by people of this state migrating to central Brazil. Human migration was common during the last 50 years due to the rapid development of Goiás. This assumption that domestication resulted from hybridization and not by selection from any wild species, has been referred to by Rogers (1963), who used the expression ‘species complexity’. Allem (1994) proposed that cassava arose from M. flabellifolia, which has a strong morphological similarity to M. esculenta, but this seems extremely unlikely. In the same paper, Allem identified a second species. He gave no evidence in support of this proposition. On the contrary, the recent molecular study of Hayson et al. (1994) showed that M. flabellifolia cannot be considered as a valid wild Manihot species, as it is no more than an escapee of M. esculenta adapted to certain environmental niches. If it was left to further generations it would segregate. This proposition may be applied to M. peruana also. Both these forms may be variants or escapees of M. esculenta itself, and not its ancestor. In order to cytogenetically confirm the ancestral nature of a putative ancestor, it must be hybridized and the hybrid should show synapsis and chromosome pairing. In ideal situations there should be ethnobotanical evidence that the putative ancestor was cultivated by certain ethnical groups and archeological evidence must show how it evolved during a course of time. Allem (1994) presented no such evidence. The place of domestication still needs some discussion. I have already stated that this crop has not evolved from wild species by means of natural selection. Studying the history of ethnological groups in Brazil and their migrations throws some light on this subject. The ‘Arual’ people, who lived in the northern part of the Amazon more than a thousand years ago, grew cassava and practiced a developed agriculture (Schmidt, 1951; MacNeish, 1964). Their name in the Indian language means ‘people who eat tubers’. It is seen from numerous reports (Schmidt, 1951; Sauer, 1952) that they cultivated cassava many centuries before Columbus. The Aruak were obliged to migrate in the 11th century to Central America, crossing the Caribbean sea and first settling in the West Indies. Cassava carried by the Aruak to Mexico would be expected to have hybridized with local wild species, creating a center of diversity in this region. This is the most probable explanation for the formation of a center of diversity in Mexico. The history of the Aruak migration to the Bolivian Planalto and to central Brazil would explain the existence of the two centers of diversity in these regions. The northeastern Brazilian center of diversity is believed to be result of migration of the Tupi-Guarani group (Schmidt, 1951). We need to determine which of the four centers constitutes the primary center of diversity of Manihot. I propose that central Brazil, with its large number of Manihot species, is this primary center. Stebbins (1950) explanations of Vavilov’s interpretation of diversity patterns may be useful here. Vavilov’s concept is an elaboration of Willy’s Agend-Area hypothesis, i.e., the longer a given biological entity occupies an area, the greater the variability of Manihot species and the more likely that it constitutes the primary center of diversity. This assumption finds support in the fact that species that exhibit the most primitive characters are confined to this region: M. stipularis, M. pusilla, M. longipetiolata with their dioecious inflorescences and M. stricta, M. purpureo-costata and M. salicifolia with their nonlobed and sessile leaves. RELATIONSHIPS BETWEEN MANIHOT SPECIES Rogers and Appan (1973) recognized 98 Manihot species. Only one species, Manihotoides pausiflora, is known in the closest related genus, Manihotoides. Several of its attributes are not found in any Manihot species, such as a unifloral inflorescence, which is a primitive character compared with the multi-flowered inflorescences in Manihot. Manihotoides has its leaves born at the apex of short, condensed stems arising from branchlets. Rogers and Appan (1973) divided Manihot species into 19 sections, varying from trees in the section Glaziovianmae to subshurbs, nearly acaulescent, in the section Stipularis. The species in this latter section are also characterized by being more dioecious than monoecious, a condition reversed in all other Manihot species. Other sections, such as Tripartitae and Graciles, are perennial subshurbs, with large woody roots. Their stems frequently die back to the root crown in response to dry periods or fires. All the Manihot species are native to tropical regions of the New World, particularly Brazil and Mexico. Nassar (1978b) identified four centers of diversity for these species: Mexico, and Northeast, central and southwest Brazil. Microcenters of diversity of these species exist within Central Brazil, where large numbers of species are concentrated in small areas, less than 50 km in diameter (Nassar 1978b,c,d,e, 1979a,b, 1982). Nassar has attributed the formation of these microcenters to the occurrence of frequent hybridization among the species, and, the heterogeneous topography of their habitats, which tend to isolate small gene pools, leading to specification. Tree-like species, such as M. glaziovii and M. pseudogalziovii, are found in northeastern Brazil, while short species and subshurbs are found in central Brazil. Natural hybridization occurs among wild Manihot species and between these species and cassava (Nassar, 1979a,b, 1984, 1989). Barriers within the genus appear to be weak, indicating recent evolution of the group. All wild Manihot species that have been examined cytogenetically have a chromosome number of 2n = 36 (Nassar, 1978a). In spite of this high chromosome number, Manihot species behave meiotically as if they were diploids, so it is believed that polyploidization anticipated the emergence of the whole group and is responsible for their rapid speciation and for the weak interspecific barriers, facilitating interspecific hybridization. Heterozygous gene pools are thus created, followed by differentiation; thus beginning a cycle of hybridization, followed by speciation. Harlan (1970) gave convincing examples of this cycle of polyploidization and hybridization in barley and wheat. Nassar (1984) reported frequent hybridization between M. reptans Pax and M. alutacea in natural sympatric habitats, where their population boundaries overlap. Morphological markers of leaf color and bract size have been used to identify this interspecific hybridization. The range of M. reptans has expanded over the last 100 years (Nassar, 1984). This is attributed to the continuing natural crossing taking place among Manihot species. Natural hybridization of M. reptans with other species has allowed its ecotypes to colonize areas where (pure) M. reptans had been unable to do so before. This phenomenon has also been noted in the other species, such as M. caerulescens (Nassar, 1979b, 1982). The marker characters lobe shape, presence of stem nodes, flower disc color, fruit color and fruit shape have been identified in controlled crosses between cassava and wild Manihot species (Nassar, 1989), as well as in natural hybrids between cassava and different species (Nassar, 1984). These characters have been used to identify hybridization. Interspecific hybrids of cassava with M. glaziovii, M. pseudoglaziovii, M. aesculifolia, M. pilosa, M. corimbiflora, M. dichotoma, M. pohlii, M. neusana and M. anomala were obtained by Nassar through controlled crosses, although their frequency was low (Nassar, 1980a, 1989; Nassar et al., 1986). The meiotic behavior of several hybrids (cassava with M. neusana and cassava with M. pseudoglaziovii) has been studied, and low hybrid fertility was found between these species and cassava (Nassar, 1991; Nassar et al., 1996a). Grattapaglia et al. (1986) conducted a biosystematic analysis of 19 wild Manihot species, based on soluble seed protein patterns. Several species were found to be highly similar, for example: M. fruticolosa, M. pentaphyla, M. pilosa and M. corymbiflora. These results correlated well with the taximetric analysis made by Rogers and Appan (1973). M. pilosa and M. corymbiflora are the species most similar to M. esculenta. Prophyle analysis confirmed the introgression between M. caerulescens and M. esculenta. These two varieties of cassava were found to have similarity of 78% with two species of the section Glaziovinae: M. glaziovi and M. pseudoglaziovii. The high similarity between M. pilosa, M. corymbiflora and M. esculenta led to the belief that the former two species made up the hybrid complex from which the cultigen had originated (Nassar, 1978b). The high similarity between species in various sections is indicative of their recent speciation and agrees with the taxonomic classification. Genetically stated, they probably share the same gene pool. SPECIATION WITHIN THE GENUS: THE CASE OF M. REPTANS The collection of M. reptans made by Ule in 1892 was confined to the northern border of Minas Gerais, close to Goiás (Rogers and Appan, 1973), but I have found it now to be widespread over most of Goiás. During the last 80 years this species may have expanded its geographical distribution and ecological range through induced genetic variation and interspecific hybridization. In our samples of M. reptans, leaf shape varies widely, reflecting the extent of hybridization with other Manihot species. For example, M. reptans from Goiás Velho is distinguished by bright red leaf veins, a characteristic of the native M. alutacea. M. reptans was identified by its characteristic growth habit, flower and inflorescence morphology. Donations of genes from different species adapted to different environments could be the reason this species expanded rapidly throughout the State of Goiás. Harlan (1961) gave the example of Helianthus annuus (the annual sunflower), which acquired a vast gene pool due to the formation of hybrids with at least six other Helianthus species. HYBRIDIZATION OF CASSAVA AND WILD RELATIVESNassar (1989) hybridized two Manihot species, M. neusana and M. anomala, with cassava, M. esculenta, through controlled crosses using insects. The following marker characters were used to identify possible hybrids: variegated fruit color dominant to smooth, red flower disk dominant to yellow, setaceous bracteole dominant to foliaceous, and noded stem dominant to smooth. Growth habit, height, stem texture, and tuber formation were also recorded. Interspecific hybrids were identified by the dominant markers from cassava; noded stem, setaceous bracteole, ribbed fruit, and tuberculated root (Table 1). We can conclude from these results that glabrous stem, setaceous-foliaceous bracteoles, red-creamy color of flower disks, variegated-fruit green color, and ribbed versus nonribbed fruit are simple marker characters that can be used to determine whether interspecific barriers between Manihot species can be overcome by using pollen of diverse sources and insect pollinators. Based on this evidence it appears that the genetic barriers between cassava and other Manihot species are weak, and hence recently evolved. They may have arisen not as a primary isolating event, but secondarily after geographic isolation. Nassar (1978b) postulated that cassava is an interspecific hybrid that appeared by domestication within the last 2000 years ago.
APOMIXIS AND ITS ROLE IN MANIHOT SPECIATION Apomixis occurs frequently in Manihot species. This may facilitate rapid speciation in this genus. Polyploidy may offer the heterozygosity necessary for initial speciation. Hybrids produced in the future can be maintained through apomixis. Nassar (1995), Nassar and Freitas (1997), Nassar et al. (1998) found apomixis and studied its anatomic nature and cytogenetic basis in cassava. Putative apomixis was detected at a rate of 3.13%. The anatomical study showed that it is due to the formation of aposporic embryos. Apparently, apomixis enabled interspecific hybrids to maintain their heterogenic structure in certain environmental niches, and consequently contributed to the formation of a number of species in this genus. UNREDUCED GAMETES AND THEIR ROLE IN THE POLYPLOIDIZATION OF MANIHOT Nassar (1991) obtained polyploid types, which were found to be produced by unreduced gamete fertilization. The formation of unreduced microspores most likely played a major role in the origin of polyploidy in Manihot, which may be responsible for the considerable variability in this genus and for the development of hybrids. To study the occurrence of unreduced gametes in cassava, Vasquez and Nassar (1994) used nine cassava clones. Out of these nine, 8 had a normal metaphase, with complete pairing and the formation of 18 bivalents. The ninth clone, popularly known as Chioriqui, an indigenous Costa Rican clone, was found to have a sectorial chimera in the inflorescence. One sector of the inflorescence developed flowers with a normal metaphase I and complete pairing of chromosomes, while the other sector was highly sterile and had a high percent of dyads. This may be evidence for meiotic restitution in cassava, due to gene mutation, as well as for meiotic irregularity. Nassar et al. (1996a) studied 11 interspecific hybrids of cassava with wild relatives. They detected high frequencies of dyad formation, such as 3.7% in progeny of the hybrid of M. glaziovii with M. esculenta. They concluded that unreduced gametes have been responsible for polyploidization in this group in the past and that polyploidy has contributed to the success and the reproduction of sterile interspecific hybrids by making them fertile. We conclude that three processes have contributed to the evolution of the Manihot group: interspecific hybridization, polyploidy and apomixis. Interspecific hybridization provided the genetic variability necessary for initial speciation, while polyploidy contributed to the restoring of fertility to sterile hybrids. The role of apomixis was to maintain and perpetuate sterile hybrids adapted to certain environmental niches. Since apomixis is facultative, natural selection would also act on sexually reproduced genotypes, accelerating the evolution process. ACKNOWLEDGMENTS The living collection of Manihot species used in these studies was established at the Universidade de Brasília with the help of the International Development Research Center (IDRC) Ottawa, Canada, for which the author is very grateful. REFERENCES Abraham, A. (1975). Breeding of tuber crops in India. Indian J. Genet. 17: 212-217. Allem, A.C. (1994). The origin of Manihot esculenta Crantz 1994. Gen. Res. Crop Evol. 41: 133-150. Bolhuis, G.G. (1953). A survey of some attempts to breed cassava varieties with a high content proteins in the roots. Euphytica 20: 107-112. Cruz, N.D. (1968). Citologia no Gênero Manihot Adans. Determinação do número de cromossomos em algumas espécies. An. Acad. Bras. Cienc. 40: 81-95. Dobzhansky, T. (1973). Genética do Processo Evolutivo. Tradução de Celso Abbade Mourão. Polígono Editora, São Paulo, SP, Brazil, pp. 453. Grattapaglia, D.E., Nassar, N.M.A. and Dianese, J.C. (1986). Biosistemática de espécies brasileiras do Gênero Manihot baseada em padrões de proteína da semente. Cien. Cult. 19: 294-300. Harlan, J. (1961). Geographic Origin of Plants Useful to Agriculture: Germplasm Resources. AAAS, New York, NY, USA, pp. 3-9. Harlan, J.R. (1970). Evolution of cultivated plants. In: Genetic Resources in Plants - Their Exploration and Conservation (Frankel, O.H. and Bennet, E., eds.). Blackwell Sci. Publ., Oxford, England. Hayson, H.R., Chan, T.L. and Hugs, M.A. (1994). Phylogenetic relationships of Manihot species revealed by restriction fragment length polymorphism. Euphytica 76: 227-234. Jennings, D.L. (1959). Manihot melanobasis Muell. Arg. - a useful parent for cassava breeding. Euphytica 8: 157-162. Lanjouw, J. (1939). Two interesting species of Manihot L. from Surinam. Rec. Trav. Bot. Neerl. 36: 542-549. MacNeish, R.S. (1964). Ancient Mesoamerican civilization. Science 143: 531-553. Nassar, N.M.A. (1978a). Genetic resources of cassava: Chromosome behavior in some Manihot species. Indian J. Genet. 38: 135-137. Nassar, N.M.A. (1978b). Conservation of the genetic resources of cassava Manihot esculenta; determination of wild species localities with emphasis on probable origin. Econ. Bot. 32: 311-320. Nassar, N.M.A. (1978c). Some further species of Manihot with potential value to cassava breeding. Can. J. Plant Sci. 58: 915-916. Nassar, N.M.A. (1978d). Microcentres of wild cassava Manihot spp. diversity in central Brazil. Turrialba 28: 345-347. Nassar, N.M.A. (1978e). Wild Manihot species of Central Brazil for cassava breeding. Can J. Plant Sci. 58: 257-261. Nassar, N.M.A. (1979a). A study of the collection and maintenance of the germplasm of wild cassavas, Manihot spp. Turrialba 29: 221-221. Nassar, N.M.A. (1979b). Three Brazilian Manihot species with tolerance to stress conditions. Can. J. Plant Sci. 59: 533-555. Nassar, N.M.A. (1980a). Attempts to hybridize wild Manihot species with cassava. Econ. Bot. 34: 13-15. Nassar, N.M.A. (1982). Collecting wild cassava in Brazil. Indian J. Genet. 42: 405-411. Nassar, N.M.A. (1984). Natural hybrids between Manihot reptans Pax and Manihot alutacea Rogers and Appan. Can. J. Plant Sci. 64: 423-425. Nassar, N.M.A. (1989). Broadening the genetic base of cassava, Manihot esculenta Crantz, by interspecific hybridization. Can. J. Plant Sci. 69: 1071-1073. Nassar, N.M.A. (1991). Production of triploid cassava, Manihot esculenta Crantz, by hybrid diploid gametes. Field Crop Res. 13: 173-182. Nassar, N.M.A. (1995). Development of cassava interspecific hybrids for savanna (cerrado) conditions. J. Root Crops 22: 9-17. Nassar, N.M.A. and Freitas, M. (1997). Prospects of polyploidizing cassava, Manihot esculenta Crantz, by unreduced microspores. Plant Breed. 116: 1050-1055. Nassar, N.M.A., Silva, J.R. and Vieira, C. (1986). Hibridação interespecífica entre mandioca e espécies silvestres do Manihot. Cienc. Cult. 33: 1050-1055. Nassar, N.M.A., Nassar, H.N.M., Carvalho, G.G. and Vieira, C. (1996a). Induction of a productive aneuploid in cassava, Manihot esculenta Crantz. Braz. J. Genet. 19: 123-125. Nassar, N.M.A., Vieira, M.A., Vieira, C. and Grattapaglia, D. (1998). Molecular and embryonic evidence of apomixis in cassava. Euphytica 102: 9-13. Nichols, R.F.W. (1947). Breeding cassava for resistance. East. Afr. Agric. J. 12: 184-194. Rogers, D.J. (1963). Studies on Manihot esculenta Crantz and related species. Bull. Torrey Bot. Club 99: 43-54. Rogers, D. and Appan, C. (1973). Manihot, Manihotoides, Euphorbiaceae, Flora Neotropica. Hafner Press, New York, NY, USA. Rogers, D.S. and Fleming, H.S. (1973). A monograph of M. esculenta. Econ. Bot. 27: 1-113. Sauer, C.O. (1952). Agricultural Origins and Dispersal. MIT Press, Cambridge, MA, USA. Schmidt, C.B. (1951). A mandioca, contribuição para o conhecimento de sua origem. Boletim da Agricultura No. 1:1, Diretoria de Publicidade Agrícola, Secretaria da Agricultura, Indústria e Comércio, São Paulo, SP, Brazil. Stebbins, G.L. (1950). Variation and Evolution in Plants. Columbia Univ. Press, New York, NY, USA, pp. 420. Vasquez, N. and Nassar, N.M.A. (1994). Unreduced microspores in cassava, Manihot esculenta Crantz clones. Indian J Genet. 54: 436-441. Vavilov, N.I. (1951). Phytogeographic basis of plant breeding. The origin, variation immunity and breeding of cultivated plants. Chron. Bot. 13: 1-366. Vries, C.A., Ferweds, J.D. and Flash, M. (1976). Choice of crops in relation to actual and potential production in tropics. Neth. J. Agric. Sci. 15: 241-246. |