ABSTRACT. We developed a bioassay to measure the flying power of drone, in order to determine which drones could reach a drone congregation area. A wind tunnel was used to test unparasitized drones and drones slightly parasitized by one or two mites during pupal development, and counts were made of the number of spermatozoa that they produced. Drones parasitized with one mite flew as long as control drones ( Key words: Apis mellifera, Drone flying power, Sperm production, Pupal infestation, Varroa destructor INTRODUCTION In honey bees, the male mating strategy entails flights to distant drone congregation areas (DCAs) where drones may meet virgin queens (Ruttner and Ruttner, 1972; Gries and Koeniger, 1996). Mating occurs in flight and therefore demands good flying power. Those males fit enough to reach a DCA and to chase a queen for copulation can be regarded as potential mates. In addition, the number of ejaculated spermatozoa is of paramount importance to male fitness because only a fraction of its sperm, transmitted in a multiple mating sequence, will later migrate into the queen’s spermatheca (Woyke and Jasinski, 1973) and become available for egg insemination. In a series of studies on the effects of Varroa destructor parasitism during the pupal stage of Apis mellifera drones, a significant reduction in drone body weight at emergence was found, even when only one female mite had invaded a drone brood cell (Duay et al., 2002). Drone life expectancy was found to be reduced when there was multiple infestation, and only those drones with less than two adult female mites in their brood cells survived long enough to reach sexual maturity (Duay, 2002). Sylvester et al. (1999) recorded that drones from heavily infested colonies could mate with honey bee queens, but they did not show whether these were unparasitized or parasitized individuals. D. Bubalo, H. Pechhacker, A. Willam, N. Kezic and D. Sulmanovic (unpublished data) also found that drones from such colonies reached DCAs, but the number of their spermatozoa was reduced. However, the relation between the level of pupal infestation and flight performance has remained unclear. We studied this question by measuring the capacity of drones to fly in a wind tunnel. In addition, we determined the number of spermatozoa in the same drones. MATERIAL AND METHODS In the 2001 season, we collected drone combs with sealed brood cells from Carniolan Apis mellifera colonies in the apiary of the University of Tübingen, and placed them in an incubator at 34°C. Shortly before emergence, we opened and inspected the cells for the presence of Varroa destructor mites. We marked uninfested drones and those from cells containing one or two old female mites, using colored number tags (Opalithplättchen). One hour later, we introduced them into a queenright observation hive comprised of four Langstroth frames. We placed a vestibule closed with a queen excluder screen at the hive entrance (Tozetto et al., 1997). In June and early July we performed the flight performance tests between 13:30 and 17:00 h on warm, sunny days, with drones older than 12 days that were attempting to depart from their hive. In previous studies at Tübingen, drone orientation flights were observed about 8 days after emergence and mating flights at an age of 12 days or more (Tozetto et al., 1997). We tested 68 drones that had emerged from unparasitized cells, 53 from brood cells infested with one adult mite and 31 from cells infested with two adult mites. We took the drones at random out of the vestibule, one at a time, and tested them immediately for flight performance in a wind tunnel. The test system consisted of a transparent plexiglass tube 2.20 m long with a 0.42 m in inner diameter (Figure 1). At one open end, a ventilator blew air into the tunnel. We found that the drones could fly and maintain a constant position with a wind speed of 4 m/s, which is approximately 15 km/h, determined by an anemometer (Eschenbach W10). We positioned a wire cage at the other end, through which we could introduce the drones into the tunnel. In order to keep the drones flying straight, we covered the wire cage with a dark cloth to ensure that drones flew towards the other end of the tunnel. If a drone landed, we started him again immediately by hand. We recorded the number of such stopovers and the duration of all the flight periods until the drone was unable to fly anymore. We made the measurements with a chronograph (Oregon Scientific WB-388), to the nearest second.
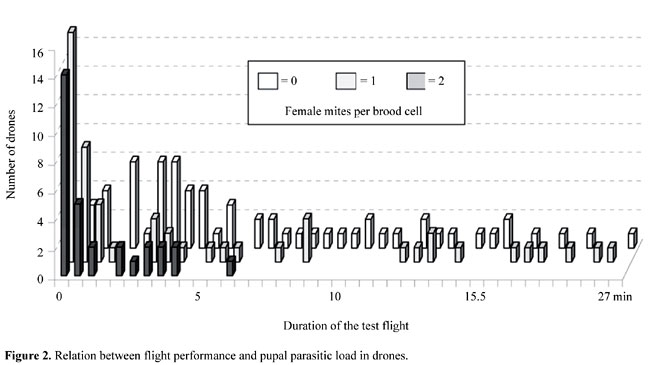
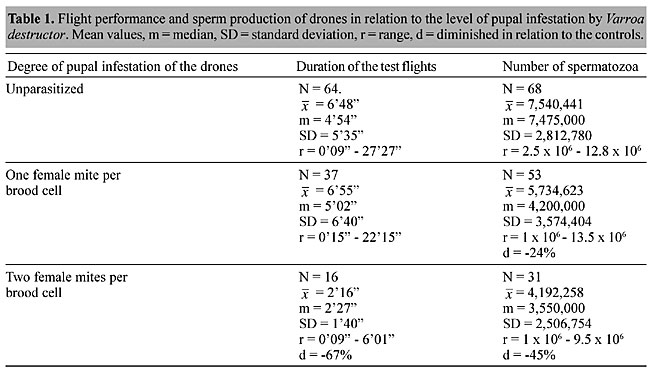
Immediately after we had tested a drone, we put it into a transport cage (11 x 13 x 6 cm), with sugar candy. On the same day we determined the number of spermatozoa that it contained. After dissecting the seminal vesicles, the spermatozoa were counted according to Camargo (1975), using a Neubauer cell chamber and ethylene glycol diluted in distilled water. We used c2 test with the Yates correction to compare differences between groups, and the Mann-Whitney U-test and the Kruskal-Wallis H-test to compare differences in mean values in pairs and between groups. We also examined the relation between flight duration and the number of sperm in each drone by Spearman rank correlation (Bühl and Zöfel, 1998). RESULTS Some drones did not fly at all (Figure 2), especially individuals that had been infested by Varroa destructor mites as pupae. The fraction of non-flying drones was significantly higher in males previously infested by one mite or two mites than in unparasitized males (c2 = 17.5, d.f. = 1, P<0.001). The maximum test flight duration was 27 min, found for one control drone (Figure 2). One drone from the group parasitized by one mite flew 22 min. However, in the group of drones previously infested by two mites, the longest test flight recorded lasted only 6 min. The mean total duration of the active flights performed by the previously uninfested drones (6 min, 48 s), was significantly greater than in those infested by two mites (2 min, 16 s) (Mann-Whitney U-test = 238.5, Z = -3.29, P<0.001). However, there was no significant difference between drones infested with one mite and control drones (Mann-Whitney U-test = 1091.0, Z = -0.656, P = 0.512).
The mean number of spermatozoa in the control drones was 7,540,441; this was significantly higher than in the groups of parasitized drones (Kruskal-Wallis H-test: c2 = 29.1, d.f. = 2, P<0.001). We also found a significant positive correlation (P<0.01) between the total duration of the test flights and the number of spermatozoa in the controls (r = 0.53; N = 64) as well as in the drones infested with one (r = 0.43; N = 37) and two mites (r = 0.54; N = 16), respectively (Table 1).
DISCUSSION In Central Europe, drone orientation flights last 10 min or less and mating flights average 20-25 min (Drescher, 1969; Whitherell, 1972; Berg, 1988; Koeniger, 1988). Mating flights are performed on warm sunny days with winds with a maximum speed of 4 m/s (Lensky and Demter, 1985). Since these same conditions were provided in our flight test, in which some control drones were able to fly for almost half an hour, we assume that our bioassay data give a realistic indication of the flight performance of individual drones. We conclude that most of the drones parasitized by Varroa mites during pupal infestation will unlikely be able to reach a DCA. Similar conclusions were also reached by Pechhacker (1998) and Sylvester et al. (1999), although no relation was made with the degree of individual pupal infestation. In addition, the diminished life expectancy of parasitized drones (Rinderer et al., 1999; Collins and Pettis, 2001, and P.R. Duay, unpublished results) and the probable disorientation during early orientation flights (D. Bubalo, H. Pechhacker, A. Willam, N. Kezic and D. Sulmanovic, unpublished data) will also reduce the number of Varroa-parasitized drones that can be regarded as potential mates. Even if a drone has reached a DCA, it must be capable of chasing the queen in a high-speed flight, in order to have a chance to copulate with her (Gries and Koeniger, 1996). Our data suggest that few of the mite-parasitized drones would be able to do so. For the first time we have demonstrated a correlation between flight performance and the number of spermatozoa in individual drones. The observations of Rinderer et al. (1999) and D. Bubalo, H. Pechhacker, A. Willam, N. Kezic and D. Sulmanovic (unpublished data) point in the same direction. However, Collins and Pettis (2001) did not find diminished sperm production in Varroa-parasitized drones. Whether the spermatozoa of parasitized males also have deficiencies in their fertilization capacity, as suggested by Del Cacho et al. (1996), needs further investigation. In conclusion, varroosis is assumed to affect the mating behavior of drone honey bees because of its strong negative influence on male fitness. There may be some contribution of parasitized drones to reproduction. However, it seems to be restricted to a small proportion of the adult drones that have been infested as pupae by only one Varroa mite. Breeding programs for varroosis tolerance could use this biotest to select sexually mature parasitized drones when they collect semen for artificial insemination of queens. ACKNOWLEDGMENTS The plexiglass tube was kindly donated by Röhm & Haas GmbH, Darmstadt, Germany. The bees used for this study were reared and provided by Imkermeister Andreas Oelkrug. We thank Robert Paxton and Peter Rosenkranz for critical reading the manuscript and Beatriz Varillas for technical help with the time recordings. P. Duay gratefully acknowledges a doctoral scholarship from the DAAD. REFERENCES Berg, S. (1988). Größenabhängige Flugdauer beim Paarungsflug der Drohnen (Apis mellifera L.). In: Biona Report 6, The Flying Honeybee (Fischer, N.W.G., ed.). Verlag G.m.b.H., Stuttgart, Germany, pp. 43-50. Bühl, A. and Zöfel, P. (1998). SPSS, Praxisorientierte Einführung in die moderne Datenanalyse. Addison Wesley Longman Verlag G.m.b.H., München, Deutschland. Camargo, C.A. (1975). Biology of the spermatozoon of Apis mellifera, 1. Influence of diluents and pH. J. Apicult. Res. 14: 113-118. Collins, A.M. and Pettis, J.S. (2001). Effect of Varroa infestation on semen quality. Am. Bee J. 141: 590-593. Del Cacho, E., Marti, J.I., Josa, A., Quílez, J. and Sánchez-Acedo, C. (1996). Effect of Varroa jacobsoni parasitization in the glycoprotein expression on Apis mellifera spermatozoa. Apidologie 27: 87-92. Drescher, W. (1969). Die Flugaktivität von Drohnen der Rasse (Apis mellifera carnica L.) und (A.m. ligustica L.) in Abhängigkeit von Lebensalter und Witterung. Z. Bienenforsch 9: 390-409. Duay, P.R., De Jong, D. and Engels, W. (2002). Weight loss in drone pupae infested by Varroa destructor. Apidologie (in press). Gries, M. and Koeniger, N. (1996). Straight forward to the queen: pursuing honeybee drones (Apis mellifera L.) adjust their body axis to the direction of the queen. J. Comp. Physiol. A. 179: 539-544. Koeniger, G. (1988). Mating flights of honey bee drones (Apis mellifera L.). A film documentation. In: Biona Report 6, The Flying Honeybee (Fischer, N.W.G., ed.). Verlag G.m.b.H., Stuttgart, Germany, pp. 29-34. Lensky, Y. and Demter, M. (1985). Mating flights of the queen honeybee (Apis mellifera) in subtropical climate. Comp. Biochem. Physiol. 81A: 229-241. Pechhacker, H. (1998). Paarungskontrolle und Belegstellen. Allg. Dtsch. Imker Z. 8: 14-15. Rinderer, T.E., De Guzman, L.I., Lancaster, V.A., Delatte, G.T. and Stelzer, A.J. (1999). Varroa in the mating yard: I. The effects of Varroa jacobsoni and Apistan on drone honey bees. Am. Bee J. 139: 134-139. Ruttner, H. and Ruttner, F. (1972). Untersuchungen über Flugaktivität und das Paarungsverhalten der Drohnen. 5. Drohnensammelplatz und Paarungsdistanz. Apidologie 3: 203-232. Sylvester, H.A., Watts, R.P., De Guzman, L.I., Stelzer, A.J. and Rinderer, T.E. (1999). Varroa in the mating yard: II. The effects of Varroa and fluvalinate on drone mating competitiveness. Am. Bee J. 139: 225-227. Tozetto, S.O., Rachinsky, A. and Engels, W. (1997). Juvenile hormone promotes flight activity in drones (Apis mellifera carnica). Apidologie 28: 77-84. Whitherell, P.C. (1972). Flight activity and natural mortality of normal and mutant drone honeybees. J. Apicult. Res. 3: 17-23. Woyke, J. and Jasinski, Z. (1973). Influence of external conditions on the number of spermatozoa entering the spermatheca of instrumentally inseminated honeybee queens. J. Apicult. Res. 12: 145-151. |