|
Genotoxic evaluation of the organophosphorous pesticide temephos
ABSTRACT. The chemical compound temephos (0,0,0',0'-tetrametyl-0,0'-thiodi-p-phenylene phosphorothioate) is an organophosphorous pesticide that has been used in Brazil since 1967 in control campaigns against the mosquito Aedes aegypti, the vector of dengue and yellow fever. We used single cell gel electrophoresis (SCGE), SOS/umu and Ames/Salmonella assays to test the toxicity and mutagenicity of temephos. Temephos was genotoxic in the SCGE assay, inducing severe DNA lesions (type IV lesions) at doses above 1.34 mM. It was mutagenic, but not toxic, in the SOS/umu assay to Escherichia coli strain PQ37, but not to PQ35, at concentrations above 1.33 mM, particularly when the S9 mixture was not used in the assay. Temephos was not mutagenic in the Ames assay with S. typhimurium strains TA97, TA98, TA100 and TA102, both with and without metabolic activation. However, temephos at concentrations above 3.33 mM was mutagenic to TA98NR, YG7104 and YG7108, both with and without metabolic activation. In conclusion, temephos was genotoxic and mutagenic in all the three tests used, and in two of them at concentrations similar to those routinely used to combat Aedes aegypti. Key words: Temephos, Mutagen, Ames/Salmonella test, SOS/umu assay, Comet assay
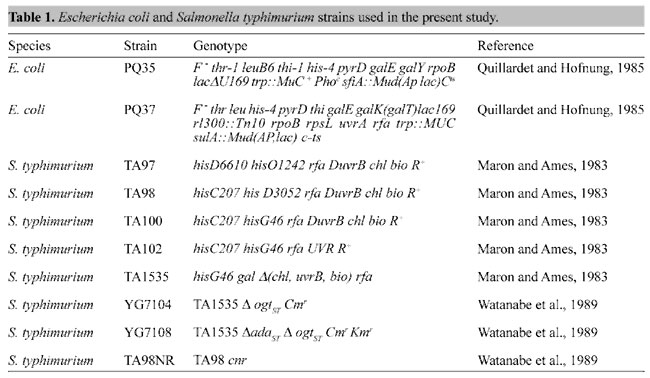
INTRODUCTION Temephos (O,O,O',O'-tetramethyl-O,O'-thiodi-p-phenylene phosphorothioate) (Abate® 4-E, Cyanamid, USA) is an organophosphorous pesticide that was widely used in Brazil from 1967 to 1998, for controlling the dengue and yellow fever vector, the mosquito Aedes aegypti. It was employed as a larvicide in stagnant water at a 1% (w/v) concentration, and in household water at a final concentration of 1 ppm (Nobre, 1998). Organophosphorous pesticides act by inhibiting acetylcholinesterase hydrolysis of acetylcholine, resulting in acetylcholine accumulation in neuromuscular synapses. The acute toxic effects of organophosphate pesticides are due to the hyperstimulation of muscarinic and nicotinic receptors, resulting in symptoms that range from increased secretions to death by respiratory depression. However, humans who ingested 256 mg temephos/person/day for 5 days or 64 mg temephos/person/day for four weeks showed no symptoms of temephos toxicity, or detectable effects on blood cholinesterase activity (Gallo and Lawrk, 1991). Temephos has been shown to be weakly mutagenic in the Ames/Salmonella test and in tests on rabbits, and on various strains of bacteria temephos was found to be nonmutagenic (U.S. Public Health Service, 1995). Since temephos has been widely used in Brazil, we decided to reevaluate its mutagenic potential by applying genotoxic tests that have not been previously employed, such as the single cell gel electrophoresis assay (Comet assay), the SOS/umu assay, and the Ames/Salmonella test, using additional bacterial strains. MATERIAL AND METHODS Reagents and Strains Eight-week-old male Wistar rats were obtained from the FIOCRUZ Central Animal Breeding Facility, Rio de Janeiro, RJ, Brazil, kept in 12-h light-dark cycles, and supplied with water and a pelleted diet (Nuvital, Nuvilab, Curitiba, PR, Brazil) ad libitum. The Escherichia coli and Salmonella typhimurium strains used in the present study (Table 1) are described elsewhere (Felzenszwalb and Alcantara Gomes, 1982; Watanabe et al., 1989; Valsa et al., 1990), and were maintained according to the revised methods of Maron and Ames (1983), and Quillardet et al. (1982). Temephos was obtained from a commercial source, in a granular 2% (w/v) formulation. One hundred grams of granular temephos was placed in 200 ml of distilled water for 14 h (protected from light). The suspension was then filtered through a 0.22-mm filter (Millipore, Brazil) and diluted to a final concentration of 21.45 mM. Single cell gel electrophoresis - (Comet assay) The Comet assay was made by incubating temephos for 90 min with total blood cells obtained from 8-week-old male Wistar rats, as previously described (da Costa Lopes et al., 2000), based on the methods of Singh et al. (1988), modified by Betti et al. (1993, 1994). Quantitative measurements of DNA breakage were made by visual scoring of 50 randomly selected cells per slide, classifying them into four categories, representing increasing degrees of damage, ranging from Comet 1, with a minimal detectable frequency of DNA lesions, as in a control group with spontaneous lesions, to a maximum length Comet (type 4) (Kovary et al., 2001).
SOS/umu assay E. coli strains (Table 1) were incubated with temephos at different concentrations at 37ºC, for 90 min, with or without 19.1 mg/plate of protein from an S9 mixture (Moltox Inc., USA). The cells (2 x 108 cells/ml) were then centrifuged and resuspended as previously described (Asad et al., 2001). The induction of the SOS response by temephos was evaluated by measuring b-galactosidase production and alkaline phosphatase activity, according to Quillardet and Hofnung (1985). Ames/Salmonella assay The Ames/Salmonella assay was performed according to Maron and Ames (1983), with slight modifications. Temephos was incubated for 90 min at 37ºC with different strains of S. typhimurium (Table 1), with or without 19.1 mg/plate mg of S9 mixture protein (Moltox Inc., USA). Statistical analysis Statistical analysis was made by the Student or Welch t-test, using Instat 2.01 software (GraphPad Software, CA, USA). RESULTS Temephos was tested in the Comet assay at concentrations ranging from 1.34 to 21.75 mM. When total blood cells were incubated with phosphate buffer (blank assay), no Comet type IV (severe lesion) was formed; Comet type I was the main category observed (Figure 1). Temephos produced a dose-dependent increase in type IV lesions, with a respective decrease in type I lesions. Temephos at 1.34 mM produced 22% type IV lesions, while at 21.75 mM type IV lesions increased to 48%. Temephos did not produce significant differences in type II and III lesions, when compared to the blank assay.
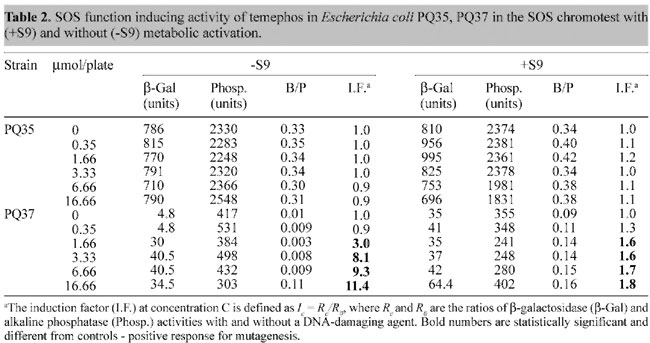
Temephos was then tested using the quantitative colorimetric SOS chromotest. This pesticide did not induce an SOS response when incubated with E. coli strain PQ35, both with and without metabolic activation (Table 2). However, incubation of temephos with strain PQ37 revealed a strong dose-dependent SOS response at concentrations ranging from 1.66 to 16.66 mM without metabolic activation. When the S9 mixture was added to the incubation mixture, the SOS response was still observed, though not as intensely.
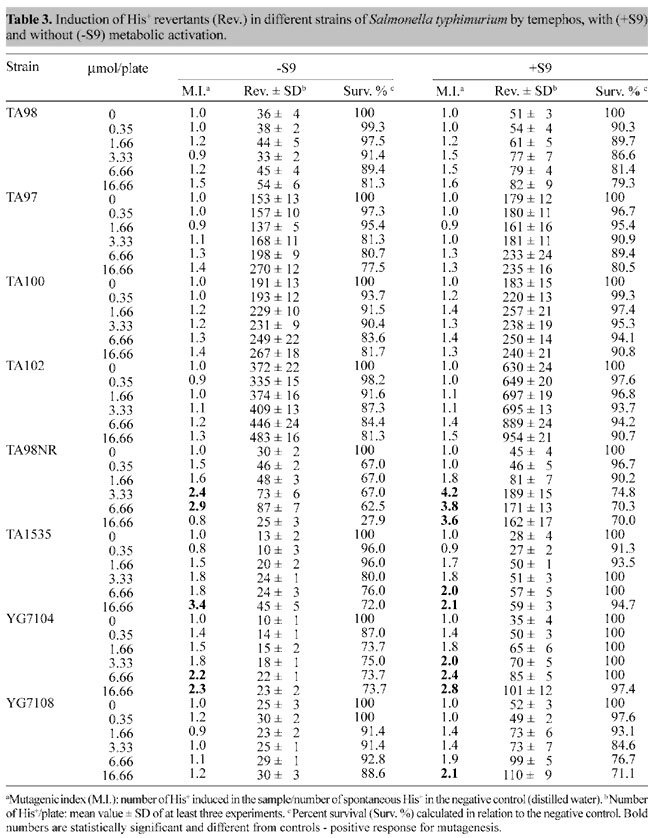
When we tested temephos in the Ames/Salmonella assay, we did not detect mutagenesis with strains TA97, TA98, TA100, and TA102 (Table 3), as also found by Bartsch et al. (1980).
However, it was mutagenic for TA98NR at concentrations above 3.33 mM, with and without metabolic activation. Based on the survival index (Table 3) temephos, without the S9 mixture, was highly toxic to TA98NR. Temephos was also mutagenic, with and without metabolic activation for strain TA1535 at concentrations above 6.66 mM, for strain YG7104 at concentrations above 3.33 mM, and at 16.66 mM for strain YG7108. However, temephos was not toxic to any of these strains at any of the concentrations used, both with and without metabolic activation. DISCUSSION The organophosphorous pesticide temephos has been widely used in Brazil as a larvicide to combat the yellow and dengue fever vector, the mosquito Aedes aegypti. Whereas yellow fever incidence in Brazil is very low and is under control, dengue fever epidemics have been constantly appearing throughout much of the country over the last two decades, affecting hundreds of thousands and causing the death of hundreds of individuals (Nobre, 1998), so there are now extensive mosquito control programs in place. We found that temephos was genotoxic in the three tests used. In the Comet assay, which identifies gross lesions in DNA caused by chemical compounds, temephos produced dose-dependent severe (type IV) lesions in the DNA of total blood cells of Wistar rats. However, the concentrations of temephos that produced these lesions were much higher than those usually applied in household water reservoirs in Brazil (2.14 mM) (Nobre, 1998). The SOS chromotest revealed that temephos is genotoxic to E. coli strain PQ37, but in this case at concentrations (above 1.66 mM) similar to those used in Brazil. The fact that temephos was capable of eliciting an SOS response to E. coli strain PQ37, but not to PQ35, suggests that the DNA damage is repaired by nucleotide excision, required by the SOS ABC protein system (Quillardet and Hofnung, 1985). Furthermore, the addition of S9 mixture to the incubation decreased the SOS response. This suggests that biotransformation of temephos leads to the formation of new compounds that are not as genotoxic as the original compound. The Ames/Salmonella assay showed that temephos was particularly mutagenic to S. typhimurium strain TA98NR, which is deficient in nitroreductase, but not to TA98, which expresses this enzyme. Apparently nitroreductase is able to metabolize temephos to products that are not mutagenic or toxic. Nitroreductase is a bacterial enzyme involved in the reductive biotransformation of certain xenobiotics containing a nitro moiety, such as nitroarenes (Watanabe et al., 1989). However, although temephos clearly was mutagenic to TA98NR, but not to TA98 (Table 2), temephos does not contain a nitro moiety, and therefore it would not be a substrate for nitroreductase. Nevertheless, biotransformation through the addition of S9 mixture to the assay decreased temephos toxicity, but not mutagenicity to TA98NR. This suggests that whereas temephos is both mutagenic and toxic, biotransformation maintains mutagenicity, but not toxicity to TA98NR. Temephos was also mutagenic to TA1535, which is useful for the detection of mutagens that do not preferentially revert TA100 (Maron and Ames, 1983), and to strains YG7104, and YG7108, that are deficient in O6-alkylguanine methyl transferase, an enzyme which repairs O6-alkylguanine, an altered component of DNA produced by certain alkylating agents such as nitrosamines (Swann, 1990). Similar to the results observed with TA98NR, the addition of the S9 mixture decreased temephos toxicity, but not its mutagenicity. A number of studies have shown that temephos is toxic for different species. Temephos given to chickens (15.3 mg/kg/day) over a 30-day period, produced leg weakness (Gallo and Lawrk, 1991). Temephos also produced a weight reduction in rats fed small doses (10 mg/kg/day) of temephos over a 2-year period. Singhal and Davies (1996) have shown that Hirudinaria manillensis exposed to doses of temephos above 1 mg/l, for 12 to 24 h, produced fewer mature oocytes and spermatids. Furthermore, temephos is not only toxic, but it is also relatively stable in the environment. Temephos applied to marsh fields in South Florida could be detected in the soil and it accumulated in oysters that extracted their food from the soil (Pierce et al., 1996). In conclusion, temephos was found to be mutagenic in the three assays, and in two of them it was mutagenic at concentrations similar to those applied in household water reservoirs; this should be taken into consideration when choosing a larvicide to combat vectors such as Aedes aegypti. We are now studying the genotoxicity and mutagenicity mechanisms of temephos. ACKNOWLEDGMENTS We are thankful to Dr. Mauro Velho from Departamento de Biologia Celular e Genética, UERJ, for donating the temephos. Research supported by FAPERJ, CNPq and SR2-UERJ. REFERENCES Asad, L.M.B.O., Medeiros, D.C., Felzenswalb, I., Leitão, A.C. and Asad, N.R. (2001). Effects of low iron conditions on repair of DNA lesions induced by Cumene peroxide in Escherichia coli. Mutat. Res. 485: 339-344. Bartsch, H., Malaveille, C., Camus, A.M., Martel-Planche, G., Brun, G., Hautefeuille, A., Sabadie, N., Barbin, A., Kuroki, T., Drevon, C., Piccoli, C. and Montesano, R. (1980). Validation and comparative studies on 180 chemicals with S. typhimurium strains and V79 Chinese hamster cells in the presence of various metabolizing systems. Mutat. Res. 76: 1-50. Betti, C., Davini, T., He, J. and Barale, R. (1993). Liquid holding effects on methylmercury genotoxicity in human lymphocytes. Mutat. Res. 301: 267-273. Betti, C., Davini, T., Giannesi, L., Loprieno, N. and Barale, R. (1994). Microgel electrophoresis assay (Comet test) and SCE analysis in human lymphocytes from 100 normal subjects. Mutat. Res. 307: 323-333. da Costa Lopes, L., Albano, F., Laranja, G.A.T., Alves, L.M., Martins e Silva, L.F., de Souza, G.P., Araujo, I.M., Nogueira-Neto, J.F., Felzenszwalb, I. and Kovary, K. (2000). Toxicological evaluation by in vitro and in vivo assays of an aqueous extract prepared from Echinodorus macrophyllus leaves. Toxicol. Lett. 116: 189-198. Felzenszwalb, I. and Alcantara Gomes, R. (1982). Reductone effect on UV-irradiated starved E. coli cells. Rev. Bras. Genet. V: 15-29. Gallo, M.A. and Lawrk, N.J. (1991). Organic phosphorus pesticides. In: Handbook of Pesticide Toxicology (Hayes,W.J. and Laws, E.R.J., eds.). Academic Press, New York, NY, USA, pp. 917-1123. Kovary, K., Louvain, T.S., Costa e Silva, M.C., Albano, F., Pires, B.B.M., Laranja, G.A.T., Lage, C.L.S. and Felzenswalb, I. (2001). Biochemical behaviour of norbixin during in vitro DNA damage induced by reactive oxygen species. Br. J. Nutr. 85: 431-440. Maron, D.M. and Ames, B.N. (1983). Revised methods for the Salmonella mutagenicity test. Mutat. Res. 113: 173-215. Nobre, A. (1998). Instruções para o pessoal de Combate ao Vetor: Manual de Normas Técnicas. 2.e. Ministério da Saúde. Secretaria Executiva. Plano Diretor de Erradicação do Aedes aegypti do Brasil, FUNASA, Brasília, DF, Brasil. Pierce, R., Henry, M., Kelly, D., Sherblom, P., Kozlowsky, W., Wichterman, G. and Miller, W. (1996). Temephos distribution and persistence in a Southwest Florida Salt Marsh Community. J. Mosq. Contr. Assoc. 12: 637-646. Quillardet, P. and Hofnung, M. (1985). The SOS chromotest: a colorimetic bacterial assay for genotoxins procedures. Mutat. Res.147: 65-78. Quillardet, P., Huisman, R. D'Ari and Hofnung, M. (1982). SOS chromotest , a direct assay of induction of an SOS function in Escherichia coli K12 to measure genotoxicity. PNAS 79: 5971-5975. Singh, N.P., McCoy, M.T., Tice, R.R. and Schneider, E.L. (1988). A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell. Res. 175: 184-191. Singhal, R.N. and Davies, R. (1996). Effects of an organophosphorus insecticide (Temephos) on gameto- genesis in the leech Hirudinaria manillensis (Hirudinidae). J. Invertebr. Pathol. 67: 100-101. Swann, P.F. (1990). Why do O6-alkylguanine and O4-alkylthymine miscode? The relationship between the structure of DNA containing O6-alkylguanine and O4-alkylthymine and the mutagenic properties of these bases. Mutat. Res. 233: 81-94. U.S. Public Heath Service (1995). Hazardous Substance Data Bank. Washington, DC, USA, 5-9. Valsa, J.O., Felzenszwalb, I., Caldeira de Araujo, A. and Alcantara Gomes, R. (1990). Genotoxic effect of a keto-aldehyde produced by thermal degradation of reducing sugars. Mutat. Res. 232: 31-35. Watanabe, M., Ishidate, M. and Nohmi, T. (1989). A sensitive method for detection of mutagenic nitroarenes: construction of nitroreductase-overproducing derivatives of Salmonella typhimurium strains TA98 and TA100. Mutat. Res. 216: 211-220. |